Abstract
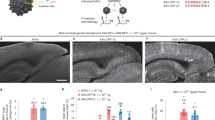
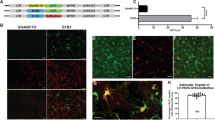
Lentiviral vectors have proven to be promising tools for transduction of brain cells in vivo and in vitro. In this study, we have examined the central nervous system (CNS) transduction efficiencies and patterns of a self-inactivating simian immunodeficiency virus (SIVmac)-derived lentiviral vector pseudotyped with glycoproteins from the vesicular stomatitis virus (VSV-G), the amphotropic murine leukemia virus (MLV4070Aenv), the lymphocytic choriomeningitis virus (LCMV-GP), the Ross River virus (RRV-GP) and the rabies virus (RV-G). All glycoproteins were efficiently incorporated into SIV virions, allowing efficient transduction of neuronal cell lines as well as of primary dissociated mouse brain cell cultures. After injection of highly concentrated vector stocks into the striatum of adult mice, quantitative analyses revealed high transduction efficiency with VSV-G pseudotypes, while LCMV-GP and RV-G pseudotypes exhibited moderate transduction efficiencies. MLV4070Aenv and RRV-GP pseudotypes, however, showed only weak levels of transduction after stereotactic injection into the brain. Regarding cell tropism in vivo, VSV-G-pseudotyped SIV vectors transduced neuronal as well as glial cells, whereas all other pseudotypes preferentially transduced neuroglial cells. In addition, we analyzed the influence of the central polypurine tract (cPPT) in context of the VSV-G-pseudotyped SIV transfer vector for infection of brain cells. Deletion of the cPPT sequence from the transfer vector decreased the in vivo transduction efficiency by fourfold, and, more importantly, this modification changed the transduction pattern, since these vectors were no longer able to infect neuronal cells in vivo. Vector injection into the brain did elicit a humoral immune response in the injected hemisphere; however, no gross signs of inflammation could be detected. Analysis of the biodistribution of the vector revealed that, besides the injected brain region, no vector-specific sequences could be detected in any of the organs evaluated. These data indicate SIV vectors as efficient gene delivery vehicles for the treatment of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Naldini L, Blomer U, Gage FH, Trono D, Verma IM . Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996; 93: 11382–11388.
Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M . Transduction of nondividing cells using pseudotyped defective high- titer HIV type 1 particles. Proc Natl Acad Sci USA 1996; 93: 15266–15271.
Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH . Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 1997; 71: 6641–6649.
Kordower JH, Bloch J, Ma SY, Chu Y, Palfi S, Roitberg BZ et al. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol 1999; 160: 1–16.
Sandrin V, Russell SJ, Cosset FL . Targeting retroviral and lentiviral vectors. Curr Top Microbiol Immunol 2003; 281: 137–178.
Stitz J, Muhlebach MD, Blomer U, Scherr M, Selbert M, Wehner P et al. A novel lentivirus vector derived from apathogenic simian immunodeficiency virus. Virology 2001; 291: 191–197.
Poeschla E, Gilbert J, Li X, Huang S, Ho A, Wong-Staal F . Identification of a human immunodeficiency virus type 2 (HIV-2) encapsidation determinant and transduction of nondividing human cells by HIV-2-based lentivirus vectors. J Virol 1998; 72: 6527–6536.
Alisky JM, Hughes SM, Sauter SL, Jolly D, Dubensky Jr TW, Staber PD et al. Transduction of murine cerebellar neurons with recombinant FIV and AAV5 vectors. Neuroreport 2000; 11: 2669–2673.
Mitrophanous K, Yoon S, Rohll J, Patil D, Wilkes F, Kim V et al. Stable gene transfer to the nervous system using a non-primate lentiviral vector. Gene Therapy 1999; 6: 1808–1818.
Cronin J, Zhang XY, Reiser J . Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 2005; 5: 387–398.
Desmaris N, Bosch A, Salaun C, Petit C, Prevost MC, Tordo N et al. Production and neurotropism of lentivirus vectors pseudotyped with lyssavirus envelope glycoproteins. Mol Ther 2001; 4: 149–156.
Kang Y, Stein CS, Heth JA, Sinn PL, Penisten AK, Staber PD et al. In vivo gene transfer using a nonprimate lentiviral vector pseudotyped with Ross River virus glycoproteins. J Virol 2002; 76: 9378–9388.
Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet 2001; 10: 2109–2121.
Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH . Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther 2002; 5: 528–537.
Wong LF, Azzouz M, Walmsley LE, Askham Z, Wilkes FJ, Mitrophanous KA et al. Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol Ther 2004; 9: 101–111.
Blomer U, Kafri T, Randolph-Moore L, Verma IM, Gage FH . Bcl-xL protects adult septal cholinergic neurons from axotomized cell death. Proc Natl Acad Sci USA 1998; 95: 2603–2608.
Pereira de Almeida LP, Zala D, Aebischer P, Deglon N . Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington's disease. Neurobiol Dis 2001; 8: 433–446.
Deglon N, Tseng JL, Bensadoun JC, Zurn AD, Arsenijevic Y, Pereira de Almeida L et al. Self-inactivating lentiviral vectors with enhanced transgene expression as potential gene transfer system in Parkinson's disease. Hum Gene Ther 2000; 11: 179–190.
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK . Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 1993; 90: 8033–8037.
DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther 2000; 2: 218–222.
Gilbert JR, Wong-Staal F . HIV-2 and SIV vector systems. Somat Cell Mol Genet 2001; 26: 83–98.
Delenda C, Audit M, Danos O . Biosafety issues in lentivector production. Curr Top Microbiol Immunol 2002; 261: 123–141.
Mangeot PE, Negre D, Dubois B, Winter AJ, Leissner P, Mehtali M et al. Development of minimal lentivirus vectors derived from simian immunodeficiency virus (SIVmac251) and their use for gene transfer into human dendritic cells. J Virol 2000; 74: 8307–8315.
Nakajima T, Nakamaru K, Ido E, Terao K, Hayami M, Hasegawa M . Development of novel simian immunodeficiency virus vectors carrying a dual gene expression system. Hum Gene Ther 2000; 11: 1863–1874.
Negre D, Mangeot PE, Duisit G, Blanchard S, Vidalain PO, Leissner P et al. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Therapy 2000; 7: 1613–1623.
Schnell T, Foley P, Wirth M, Munch J, Uberla K . Development of a self-inactivating, minimal lentivirus vector based on simian immunodeficiency virus. Hum Gene Ther 2000; 11: 439–447.
Wagner R, Graf M, Bieler K, Wolf H, Grunwald T, Foley P et al. Rev-independent expression of synthetic gag-pol genes of human immunodeficiency virus type 1 and simian immunodeficiency virus: implications for the safety of lentiviral vectors. Hum Gene Ther 2000; 11: 2403–2413.
Kitagawa R, Miyachi S, Hanawa H, Takada M, Shimada T . Differential characteristics of HIV-based versus SIV-based lentiviral vector systems: gene delivery to neurons and axonal transport of expressed gene. Neurosci Res 2007; 57: 550–558.
Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P . HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 2000; 101: 173–185.
Zennou V, Serguera C, Sarkis C, Colin P, Perret E, Mallet J et al. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat Biotechnol 2001; 19: 446–450.
Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D . Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997; 15: 871–875.
Klein D, Bugl B, Gunzburg WH, Salmons B . Accurate estimation of transduction efficiency necessitates a multiplex real-time PCR. Gene Therapy 2000; 7: 458–463.
Amado RG, Chen IS . Lentiviral vectors – the promise of gene therapy within reach? Science 1999; 285: 674–676.
Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B et al. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum Gene Ther 2002; 13: 841–853.
Jakobsson J, Ericson C, Jansson M, Bjork E, Lundberg C . Targeted transgene expression in rat brain using lentiviral vectors. J Neurosci Res 2003; 73: 876–885.
Lai Z, Brady RO . Gene transfer into the central nervous system in vivo using a recombinant lentivirus vector. J Neurosci Res 2002; 67: 363–371.
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 2000; 290: 767–773.
Azzouz M, Kingsman SM, Mazarakis ND . Lentiviral vectors for treating and modeling human CNS disorders. J Gene Med 2004; 6: 951–962.
Hatziioannou T, Cowan S, Bieniasz PD . Capsid-dependent and -independent postentry restriction of primate lentivirus tropism in rodent cells. J Virol 2004; 78: 1006–1011.
Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P . Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson's disease using GDNF. Exp Neurol 2000; 164: 15–24.
Sanders DA . No false start for novel pseudotyped vectors. Curr Opin Biotechnol 2002; 13: 437–442.
Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA 1994; 91: 7071–7075.
Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV et al. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 1998; 282: 2079–2081.
Moukhles H, Carbonetto S . Dystroglycan contributes to the formation of multiple dystrophin-like complexes in brain. J Neurochem 2001; 78: 824–834.
Miletic H, Fischer YH, Neumann H, Hans V, Stenzel W, Giroglou T et al. Selective transduction of malignant glioma by lentiviral vectors pseudotyped with lymphocytic choriomeningitis virus glycoproteins. Hum Gene Ther 2004; 15: 1091–1100.
Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L . Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 2000; 25: 217–222.
Baekelandt V, Eggermont K, Michiels M, Nuttin B, Debyser Z . Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Therapy 2003; 10: 1933–1940.
Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L et al. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther 2005; 16: 741–751.
Cosset FL, Takeuchi Y, Battini JL, Weiss RA, Collins MK . High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol 1995; 69: 7430–7436.
Beyer WR, Westphal M, Ostertag W, von Laer D . Oncoretrovirus and lentivirus vectors pseudotyped with lymphocytic choriomeningitis virus glycoprotein: generation, concentration, and broad host range. J Virol 2002; 76: 1488–1495.
Conzelmann KK, Cox JH, Schneider LG, Thiel HJ . Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 1990; 175: 485–499.
Renfranz PJ, Cunningham MG, McKay RD . Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell 1991; 66: 713–729.
Soneoka Y, Cannon PM, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM et al. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res 1995; 23: 628–633.
Yee JK, Friedmann T, Burns JC . Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 1994; 43 (Part A): 99–112.
Radad K, Gille G, Moldzio R, Saito H, Rausch WD . Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res 2004; 1021: 41–53.
Paxinos G, Franklin KBJ . The Mouse Brain in Stereotactic Coordinates: Compact, 2nd edn, Academic Press, San Diego, 2003.
Russ JC, Dehoff RT . Classical stereological measures. In: Russ JC, Dehoff RT (eds). Practical Stereology. Plenum Press: New York, 2001, pp 44–54.
Gundersen HJ, Jensen EB . The efficiency of systematic sampling in stereology and its prediction. J Microsc 1987; 147: 229–263.
Acknowledgements
We are grateful to Dr Überla, Ruhr-University Bochum, Germany, for providing plasmids pViCGΔBH and pSgpΔ2; to Dr Cosset, INSERM U758, Lyon, France, for plasmid pAXF; to Dr Burns, University of California San Diego, USA, for providing plasmid pHCMV-G; to Dr von Laer, Georg-Speyer-Haus, Frankfurt, Germany, for plasmid pHCMV–LCMV-GP; to Dr Sanders, Purdue University, West Lafayette, USA, for plasmid pRRVpcDNA3.1Zeo+; and to Dr Conzelmann, Ludwig-Maximilians-University, München, Germany for providing the Rabies virus G cDNA of strain SAD B19. We also thank Helga Petznek for help with mouse work, Doris Rosenfellner for help with histological analyses, and Christopher Krewenka from the Institute of Medical Chemistry for preparation of the primary cell cultures. Special thanks are due to Gerrit Jandl for excellent technical assistance. We very much appreciate Dr Kirsti Witter, Institute of Histology and Embryology, and Dr Herbert Weissenböck, Institute of Pathology, University of Veterinary Medicine Vienna, for helpful discussions on stereological analyses and pathological advice, respectively. Quantification of infected cells was supported by project MSM0021620819 awarded by the Ministry of Education, Youth and Sports of the Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liehl, B., Hlavaty, J., Moldzio, R. et al. Simian immunodeficiency virus vector pseudotypes differ in transduction efficiency and target cell specificity in brain. Gene Ther 14, 1330–1343 (2007). https://doi.org/10.1038/sj.gt.3302988
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302988
Keywords
This article is cited by
-
Highly efficient ex vivo lentiviral transduction of primary human pancreatic exocrine cells
Scientific Reports (2019)
-
Tropism, intracerebral distribution, and transduction efficiency of HIV- and SIV-based lentiviral vectors after injection into the mouse brain: a qualitative and quantitative in vivo study
Histochemistry and Cell Biology (2017)
-
Comparative evaluation of preclinical in vivo models for the assessment of replicating retroviral vectors for the treatment of glioblastoma
Journal of Neuro-Oncology (2011)
-
Comparative Analysis of Transduced Primary Human Dendritic Cells Generated by the Use of Three Different Lentiviral Vector Systems
Molecular Biotechnology (2011)
-
Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors
Nature Methods (2010)