Abstract
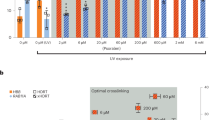
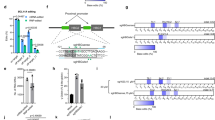
A significant level of correction of the mutation responsible for sickle cell anemia has been achieved in monkey COS-7 cells on a plasmid containing a β-globin gene fragment. The plasmid was treated in vitro with a nucleic acid ‘third strand’ bearing a terminal photoreactive psoralen moiety that binds immediately adjacent to the mutant base pair. Following covalent attachment of the psoralen by monoadduct or diadduct formation to the mutant T-residue on the coding strand, the treated plasmid was transfected into the cells, which were then incubated for 48 h to allow the cellular DNA repair mechanisms to remove the photoadducts. Upon re-isolation and amplification of the transfected plasmid, sickle cell mutation correction, as determined by sequence analysis of both complementary strands, was established in a full 1%. This result encourages extension of the approach to correct the mutation directly on the chromosome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 2000; 406: 82–86.
Persons DA . Update on gene therapy for hemoglobin disorders. Curr Opin Mol Ther 2003; 5: 508–516.
Sadelain M, Rivella S, Lisowski L, Samakoglu S, Riviere I . Globin gene transfer for treatment of the beta-thalassemias and sickle cell disease. Best Pract Res Clin Haematol 2004; 17: 517–534.
Cole-Strauss A, Yoon K, Xiang Y, Byrne BC, Rice MC, Gryn J et al. Correction of the mutation responsible for sickle cell anemia by an RNA-DNA oligonucleotide. Science 1996; 273: 1386–1389.
Li ZH, Liu DP, Yin WX, Guo ZC, Liang CC . Targeted correction of the point mutations of beta-thalassemia and targeted mutagenesis of the nucleotide associated with HPFH by RNA/DNA oligonucleotides: potential for beta-thalassemia gene therapy. Blood Cells Mol Dis 2001; 27: 530–538.
Liu H, Agarwal S, Kmiec E, Davis BR . Targeted beta-globin gene conversion in human hematopoietic CD34(+) and Lin(−)CD38(−) cells. Gene Therapy 2002; 9: 118–126.
Yin W, Kren BT, Steer CJ . Site-specific base changes in the coding or promoter region of the human beta- and gamma-globin genes by single-stranded oligonucleotides. Biochem J 2005; 390: 253–261.
Taubes G . Gene therapy. The strange case of chimeraplasty. Science 2002; 298: 2116–2120.
van der Steege GS-HP, Buys CHCM, Scheffer H, Pas HH, Jonkman MF . Persistent failures in gene repair. Nat Biotechnol 2001; 19: 305–306.
Parekh-Olmedo H, Ferrara L, Brachman E, Kmiec EB . Gene therapy progress and prospects: targeted gene repair. Gene Therapy 2005; 12: 639–646.
Barre FX, Ait-Si-Ali S, Giovannangeli C, Luis R, Robin P, Pritchard LL et al. Unambiguous demonstration of triple-helix-directed gene modification. Proc Natl Acad Sci USA 2000; 97: 3084–3088.
Wang G, Glazer PM . Altered repair of targeted psoralen photoadducts in the context of an oligonucleotide-mediated triple helix. J Biol Chem 1995; 270: 22595–22601.
Vasquez KM, Wang G, Havre PA, Glazer PM . Chromosomal mutations induced by triplex-forming oligonucleotides in mammalian cells. Nucleic Acids Res 1999; 27: 1176–1181.
Havre PA, Gunther EJ, Gasparro FP, Glazer PM . Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc Natl Acad Sci USA 1993; 90: 7879–7883.
Luo Z, Macris MA, Faruqi AF, Glazer PM . High-frequency intrachromosomal gene conversion induced by triplex-forming oligonucleotides microinjected into mouse cells. Proc Natl Acad Sci USA 2000; 97: 9003–9008.
Ingram VM . Gene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobin. Nature 1957; 180: 326–328.
Friedman M . Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci USA 1978; 75: 1994–1997.
Broitman S, Amosova O, Dolinnaya NG, Fresco JR . Repairing the sickle cell mutation. I. Specific covalent binding of a photoreactive third strand to the mutated base pair. J Biol Chem 1999; 274: 21763–21768.
Amosova O, Broitman SL, Fresco JR . Repairing the Sickle Cell mutation. II. Effect of psoralen linker length on specificity of formation and yield of third strand-directed photoproducts with the mutant target sequence. Nucleic Acids Res 2003; 31: 4673–4681.
Beal PA, Dervan PB . Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science 1991; 251: 1360–1363.
Letai AG, Palladino MA, Fromm E, Rizzo V, Fresco JR . Specificity in formation of triple-stranded nucleic acid helical complexes: studies with agarose-linked polyribonucleotide affinity columns. Biochemistry 1988; 27: 9108–9112.
Gamper HB, Stamm MR, Podyminogin MA, Meyer R . Strand Invasion of supercoiled DNA by oligonucleotides with a triplex guide sequence. J Am Chem Soc 1998; 120: 2182–2183.
Fossella JA, Kim YJ, Shih H, Richards EG, Fresco JR . Relative specificities in binding of Watson-Crick base pairs by third strand residues in a DNA pyrimidine triplex motif. Nucleic Acids Res 1993; 21: 4511–4515.
Yoon K, Hobbs CA, Koch J, Sardaro M, Kutny R, Weis AL . Elucidation of the sequence-specific third-strand recognition of four Watson-Crick base pairs in a pyrimidine triple-helix motif: T.AT, C.GC, T.CG, and G.TA. Proc Natl Acad Sci USA 1992; 89: 3840–3844.
Mergny J, Sun J, Rougee M, Montenaygarestier T, Barcelo F, Chomilier J et al. Sequence specificity in triple helix formation: experimental and theoretical studies of the effect of mismatches on triplex stability. Biochemistry 1991; 30: 9791–9798.
Cimino GD, Gamper HB, Isaacs ST, Hearst JE . Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Ann Rev Biochem 1985; 54: 1151–1193.
Broitman SL, Amosova O, Fresco JR . Repairing the Sickle Cell mutation. III. Effect of irradiation wavelength on the specificity and type of photoproduct formed by a 3′-terminal psoralen on a third strand directed to the mutant base pair. Nucleic Acids Res 2003; 31: 4682–4688.
Sears LE, Zhou B, Aliotta JM, Morgan RD, Kong H . BaeI, another unusual BcgI-like restriction endonuclease. Nucleic Acids Res 1996; 24: 3590–3592.
Harrison B, Zimmerman SB . T4 polynucleotide kinase: macromolecular crowding increases the efficiency of reaction at DNA termini. Anal Biochem 1986; 158: 307–315.
Sandor Z, Bredberg A . Repair of triple helix directed psoralen adducts in human cells. Nucleic Acids Res 1994; 22: 2051–2056.
Barre FX, Asseline U, Harel-Bellan A . Asymmetric recognition of psoralen interstrand crosslinks by the nucleotide excision repair and the error-prone repair pathways. J Mol Biol 1999; 286: 1379–1387.
Wang G, Seidman MM, Glazer PM . Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science 1996; 271: 802–805.
Tseng WC, Haselton FR, Giorgio TD . Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J Biol Chem 1997; 272: 25641–25647.
Protic-Sabljic M, Tuteja N, Munson PJ, Hauser J, Kraemer KH, Dixon K . UV light-induced cyclobutane pyrimidine dimers are mutagenic in mammalian cells. Mol Cell Biol 1986; 6: 3349–3356.
Saffran WA, Cantor CR . Mutagenic SOS repair of site-specific psoralen damage in plasmid pBR322. J Mol Biol 1984; 178: 595–609.
Acknowledgements
This work was supported by NIH Grants RO1 HL063888 and 5R33 CA088547. We are grateful to Dimitri Klimov for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is paper IV in the series ‘Repair of the Sickle Cell Mutation’, of which the last is reference 27.
Rights and permissions
About this article
Cite this article
Varganov, Y., Amosova, O. & Fresco, J. Third strand-mediated psoralen-induced correction of the sickle cell mutation on a plasmid transfected into COS-7 cells. Gene Ther 14, 173–179 (2007). https://doi.org/10.1038/sj.gt.3302850
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302850