Abstract
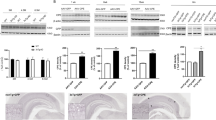
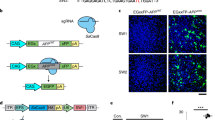
Accumulation of insoluble aggregates of amyloid-β peptide (Aβ), a cleavage product of amyloid precursor protein (APP), is thought to be central to the pathogenesis of Alzheimer's disease (AD). Consequently, downregulation of APP, or enhanced clearance of Aβ, represent possible therapeutic strategies for AD. We generated replication-defective herpes simplex virus (HSV) vectors that inhibit Aβ accumulation, both in vitro and in vivo. In cell culture, HSV vectors expressing either (i) short hairpin RNA directed to the APP transcript (HSV-APP/shRNA), or (ii) neprilysin, an endopeptidase that degrades Aβ (HSV-neprilysin), substantially inhibited accumulation of Aβ. To determine whether these vectors showed similar activity in vivo, we developed a novel mouse model, in which overexpression of a mutant form of APP in the hippocampus, using a lentiviral vector (LV-APPSw), resulted in rapid Aβ accumulation. Co-inoculation of LV-APPSw with each of the HSV vectors showed that either HSV-APP/shRNA or HSV-neprilysin inhibited Aβ accumulation in this model, whereas an HSV control vector did not. These studies demonstrate the utility of HSV vectors for reducing Aβ accumulation in the brain, thus providing useful tools to clarify the role of Aβ in AD that may facilitate the development of novel therapies for this important disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Selkoe DJ . Clearing the brain's amyloid cobwebs. Neuron 2001; 32: 177–180.
Tanzi RE, Bertram L . Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell 2005; 120: 545–555.
Citron M . Strategies for disease modification in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 677–685.
Selkoe D, Kopan R . Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 2003; 26: 565–597.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999; 400: 173–177.
Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO . Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 2003; 9: 448–452.
Dykxhoorn DM, Novina CD, Sharp PA . Killing the messenger: short RNAs that silence gene expression. Nat Rev Mol Cell Biol 2003; 4: 457–467.
Bahi A, Boyer F, Kolira M, Dreyer JL . In vivo gene silencing of CD81 by lentiviral expression of small interference RNAs suppresses cocaine-induced behaviour. J Neurochem 2005; 92: 1243–1255.
Van den Haute C, Eggermont K, Nuttin B, Debyser Z, Baekelandt V . Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Hum Gene Ther 2003; 14: 1799–1807.
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004; 10: 816–820.
Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med 2000; 6: 143–150.
Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP et al. Metabolic regulation of brain Abeta by neprilysin. Science 2001; 292: 1550–1552.
Malfroy B, Kuang WJ, Seeburg PH, Mason AJ, Schofield PR . Molecular cloning and amino acid sequence of human enkephalinase (neutral endopeptidase). FEBS Lett 1988; 229: 206–210.
Yasojima K, Akiyama H, McGeer EG, McGeer PL . Reduced neprilysin in high plaque areas of Alzheimer brain: a possible relationship to deficient degradation of beta-amyloid peptide. Neurosci Lett 2001; 297: 97–100.
Yasojima K, McGeer EG, McGeer PL . Relationship between beta amyloid peptide generating molecules and neprilysin in Alzheimer disease and normal brain. Brain Res 2001; 919: 115–121.
Helisalmi S, Hiltunen M, Vepsalainen S, Iivonen S, Mannermaa A, Lehtovirta M et al. Polymorphisms in neprilysin gene affect the risk of Alzheimer's disease in Finnish patients. J Neurol Neurosurg Psychiatry 2004; 75: 1746–1748.
Sakai A, Ujike H, Nakata K, Takehisa Y, Imamura T, Uchida N et al. Association of the neprilysin gene with susceptibility to late-onset Alzheimer's disease. Dement Geriatr Cogn Disord 2004; 17: 164–169.
Glorioso JC, Fink DJ . Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol 2004; 58: 253–271.
Krisky DM, Marconi PC, Oligino TJ, Rouse RJ, Fink DJ, Cohen JB et al. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Therapy 1998; 5: 1517–1530.
Krisky DM, Wolfe D, Goins WF, Marconi PC, Ramakrishnan R, Mata M et al. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Therapy 1998; 5: 1593–1603.
Goss JR, Goins WF, Lacomis D, Mata M, Glorioso JC, Fink DJ . Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neuropathy in streptozotocin-induced diabetes in the mouse. Diabetes 2002; 51: 2227–2232.
Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ . Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Therapy 2001; 8: 551–556.
Goins WF, Sternberg LR, Croen KD, Krause PR, Hendricks RL, Fink DJ et al. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol 1994; 68: 2239–2252.
Goins WF, Lee KA, Cavalcoli JD, O'Malley ME, DeKosky ST, Fink DJ et al. Herpes simplex virus type 1 vector-mediated expression of nerve growth factor protects dorsal root ganglion neurons from peroxide toxicity. J Virol 1999; 73: 519–532.
Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC et al. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol Ther 2004; 10: 67–75.
Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 2004; 428: 431–437.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Perez RG, Squazzo SL, Koo EH . Enhanced release of amyloid beta-protein from codon 670/671 ‘Swedish’ mutant beta-amyloid precursor protein occurs in both secretory and endocytic pathways. J Biol Chem 1996; 271: 9100–9107.
Chen X, Li J, Mata M, Goss J, Wolfe D, Glorioso JC et al. Herpes simplex virus type 1 ICP0 protein does not accumulate in the nucleus of primary neurons in culture. J Virol 2000; 74: 10132–10141.
Samaniego LA, Neiderhiser L, DeLuca NA . Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol 1998; 72: 3307–3320.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B et al. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci 2004; 24: 991–998.
Xia H, Mao Q, Paulson HL, Davidson BL . siRNA-mediated gene silencing in vitro and in vivo. Nat Biotechnol 2002; 20: 1006–1010.
Zhou H, Xia XG, Xu Z . An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi. Nucleic Acids Res 2005; 33: e62.
Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P . Alpha-synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci USA 2002; 99: 10813–10818.
Kirik D, Annett LE, Burger C, Muzyczka N, Mandel RJ, Bjorklund A . Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson's disease. Proc Natl Acad Sci USA 2003; 100: 2884–2889.
Senut MC, Suhr ST, Kaspar B, Gage FH . Intraneuronal aggregate formation and cell death after viral expression of expanded polyglutamine tracts in the adult rat brain. J Neurosci 2000; 20: 219–229.
de Almeida LP, Ross CA, Zala D, Aebischer P, Deglon N . Lentiviral-mediated delivery of mutant Huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, Huntingtin expression levels, and protein length. J Neurosci 2002; 22: 3473–3483.
Kirik D, Bjorklund A . Modeling CNS neurodegeneration by overexpression of disease-causing proteins using viral vectors. Trends Neurosci 2003; 26: 386–392.
Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN . Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem 2002; 277: 15666–15670.
Schafer SL, Vlach J, Pitha PM . Cooperation between herpes simplex virus type 1-encoded ICP0 and Tat to support transcription of human immunodeficiency virus type 1 long terminal repeat in vivo can occur in the absence of the TAR binding site. J Virol 1996; 70: 6937–6946.
Zheng H, Jiang M, Trumbauer ME, Hopkins R, Sirinathsinghji DJ, Stevens KA et al. Mice deficient for the amyloid precursor protein gene. Ann NY Acad Sci 1996; 777: 421–426.
Cao X, Sudhof TC . A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 2001; 293: 115–120.
Mattson MP . Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 1997; 77: 1081–1132.
Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW et al. Beta-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 1995; 81: 525–531.
Steinbach JP, Muller U, Leist M, Li ZW, Nicotera P, Aguzzi A . Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ 1998; 5: 858–866.
Miller VM, Gouvion CM, Davidson BL, Paulson HL . Targeting Alzheimer's disease genes with RNA interference: an efficient strategy for silencing mutant alleles. Nucleic Acids Res 2004; 32: 661–668.
Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 2003; 23: 1992–1996.
Burton EA, Huang S, Goins WF, Glorioso JC . Use of the herpes simplex viral genome to construct gene therapy vectors. Methods Mol Med 2003; 76: 1–31.
Acknowledgements
We thank Dr Edward Koo, Dr Didier Trono, Dr Neal DeLuca, Dr Ruth Perez for generously sharing plasmid, viral and cell resources, Dr Simon Watkins for assistance with micrographic imaging, Drs Julie Fradette and Paola Grandi for helpful discussion and Mingdi Zhang for technical assistance. This work was supported Public Health Service Grants DK-44935, GM-34534, HL-66949 and NS-44323 from the National Institutes of Health (JCG) and CSH was a recipient of a grant from the John and Nancy Emmerling Fund of the Pittsburgh Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, CS., Goins, W., Goss, J. et al. Herpes simplex virus RNAi and neprilysin gene transfer vectors reduce accumulation of Alzheimer's disease-related amyloid-β peptide in vivo. Gene Ther 13, 1068–1079 (2006). https://doi.org/10.1038/sj.gt.3302719
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302719
Keywords
This article is cited by
-
Illuminating the Gateway of Gene Silencing: Perspective of RNA Interference Technology in Clinical Therapeutics
Molecular Biotechnology (2012)
-
Current prospects for RNA interference-based therapies
Nature Reviews Genetics (2011)
-
The miRNA pathway in neurological and skeletal muscle disease: implications for pathogenesis and therapy
Journal of Molecular Medicine (2011)
-
Adeno-associated Virus Gene Therapy With Cholesterol 24-Hydroxylase Reduces the Amyloid Pathology Before or After the Onset of Amyloid Plaques in Mouse Models of Alzheimer's Disease
Molecular Therapy (2010)