Abstract
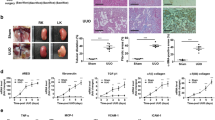
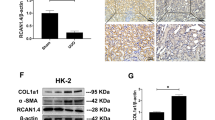
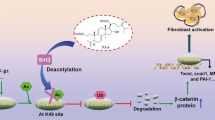
Tubulointerstitial fibrosis is the consequence of an injury characterized by the accumulation of excess collagen and other extracellular matrix components, resulting in the destruction of the normal kidney architecture and subsequent loss of function. A transcription factor Sp1, originally described as a ubiquitous transcription factor, is involved in the basal expression of extracelluar matrix genes and may, therefore, be important in fibrotic processes. Here, we report on the design of a ring-Sp1 decoy oligonucleotide, containing the consensus Sp1 binding sequence in a single decoy molecule without an open end, to create a novel therapeutic strategy for fibrosis. The ring-Sp1 decoy oligonucleotide is highly resistant to degradation by nucleases or serum compared to the conventional phosphorothioated double-stranded Sp1 decoy oligonucleotide, and effectively suppressed the expression of transforming growth factor-β1 and fibronectin, the binding of Sp1 to the promoter region of these genes, and proliferation in response to serum in normal rat kidney fibroblasts. Moreover, treatment with the ring-Sp1 decoy in vivo significantly attenuates extracellular matrix gene expression in the rat kidney in which a unilateral ureteral obstruction had been induced. These results suggest that the ring-Sp1 decoy oligonucleotide represents promising therapeutic alternative to the conventional treatment of fibrotic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- ECM:
-
extracellular matrix
- FN:
-
fibronectin
- GAPDH:
-
glyceraldehydes-3-phosphate dehydrogenase
- HVJ:
-
hemagglutinating virus of Japan
- M-Sp1 decoy:
-
mutated Sp1 decoy
- ODN:
-
oligodeoxynucleotide
- PBS:
-
phophate buffered saline
- PS-Sp1 decoy:
-
phosphorothioate Sp1 decoy
- α-SMA:
-
α-smooth muscle actin
- SSC:
-
standard saline citrate
- R-Sp1 decoy:
-
ring Sp1 decoy
- TGF-β1:
-
transforming growth factor-β1
- TNF-α:
-
tumore necrosis factor-α
- uPA:
-
urokinase-type plasminogen activator
- UUO:
-
unilateral ureteral obstruction
- VEGF:
-
vascular endothelial growth factor.
References
Klahr S, Morrissey JJ . The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int suppl 2000; 75: 7–14.
Nath KA . Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992; 20: 1–17.
Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol 1995; 130: 393–405.
Border WA, Noble NA . Transforming growth factor beta in tissue fibrosis. N Engl J Med 1994; 331: 1286–1292.
Bottinger EP, Letterio JJ, Roberts AB . Biology of TGF-beta in knockout and transgenic mouse models. Kidney Int 1997; 51: 1355–1360.
Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 1998; 53: 853–861.
Smith EA, LeRoy EC . A possible role for transforming growth factor-beta in systemic sclerosis. J Invest Dermatol 1990; 95: 125S–127S.
Quaglino DJ, Nanney LB, Ditesheim JA, Davidson JM . Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol 1991; 97: 34–42.
Roberts AB, Sporn MB . A major advance in the use of growth factors to enhance wound healing. J Am Soc Nephrol 1993; 8: 1–9.
Basile DP . The transforming growth factor beta system in kidney disease and repair: recent progress and future directions. Curr Opin Nephrol Hypertens 1996; 8: 21–30.
Klahr S, Morrissey J . Obstructive nephropathy and renal fibrosis: the role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl 2003; 87: S105–S112.
Eddy AA . Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 1996; 7: 2495–2508.
Cook T, Gebelein B, Urrutia R . Three conserved transcriptional repressor domains are a defining feature of the TIEG subfamily of Sp1-like zinc finger proteins. Ann NY Acad Sci 1999; 880: 94–102.
Goldberg HJ, Scholey J, Fantus IG . Glucosamine activates the plasminogen activator inhibitor 1 gene promoter through Sp1 DNA binding sites in glomerular mesangial cells. Diabetes 2000; 49: 863–871.
Lai A, Lee JM, Yang WM, Decaprio JA, Kaelin Jr WG, Seto E et al. RBP1 Recruits both histone deacetylase-dependent and independent repression activities to retinoblastoma family proteins. Mol Cell Biol 1999; 19: 6632–6641.
Farabaugh PJ, Vimaladitha A, Turkel S, Johnson R, Zhao H . Three downstream sites repress transcription of a Ty2 retrotransposon in Saccharomyces cerevisiae. Mol Cell Biol 1993; 13: 2081–2090.
Lin SY, Black AR, Kostic D, Pajovic S, Hoover CN, Azizkhan JC . Cell cycle-regulated association of E2F1 and Sp1 is related to their functional interaction. Mol Cell Biol 1996; 16: 1668–1675.
Karlseder J, Rotheneder H, Wintersberger E . Interaction of Sp1 with the growth- and cell cycle-regulated transcription factor E2F. Mol Cell Biol 1996; 16: 1659–1667.
Chang YC, Illenye S, Heintz NH . Cooperation of E2F-p130 and Sp1-pRb Complexes in Repression of the Chinese Hamster dhfr Gene. Mol Cell Biol 2001; 21: 1121–1131.
Park KK, Rue SW, Lee IS, Kim HC, Lee IK, Ahn JD et al. Modulation of Sp1-dependent transcription by a cis-acting E2F element in dhfr promoter. Biochem Biophys Res Commun 2003; 306: 239–243.
Kim SJ, Qnwuta US, Lee YI, Li R, Botchan MR, Robbins PD . The retinoblastoma gene product regulates Sp1-mediated transcription. Mol Cell Biol 1992; 12: 2455–2463.
Udvadia AJ, Rogers KT, Higgins PD, Murata Y, Martin KH, Humphrey PA et al. Sp-1 binds promoter elements regulated by the RB protein and Sp-1-mediated transcription is stimulated by RB coexpression. Proc Natl Acad Sci USA 1993; 90: 3265–3269.
Udvadia AJ, Templeton DJ, Horowitz JM . Functional interactions between the retinoblastoma (Rb) protein and Sp-family members: superactivation by Rb requires amino acids necessary for growth suppression. Proc Nat Acad Sci USA 1995; 92: 3953–3957.
Holmgren L, O’Reilly MS, Folkman J . Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1995; 1: 149–153.
Zhang X, Li Y, Dai C, Yang J, Mundel P, Liu Y . Sp1 and Sp3 transcription factors synergistically regulate HGF receptor gene expression in kidney. Am J Physiol Renal Physiol 2003; 284: 82–94.
Geiser AG, Busan KJ, Kim SJ, Lafyatis R, O’Reilly MA, Webbink R et al. Regulation of the transforming growth factor-beta 1 and -beta 3 promoters by transcription factor Sp1. Gene 1993; 129: 223–228.
Nehls MC, Brenner DA, Gruss HJ, Dierbach H, Mertelsmann R, Herrmanna F . Mithramycin selectively inhibits collagen-alpha 1(I) gene expression in human fibroblast. J Clin Invest 1993; 92: 2916–2921.
Hata Y, Duh E, Zhang K, Robinson GS, Aiello LP . Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J Biol Chem 1998; 273: 19294–19303.
Ishibashi H, Nakagawa K, Onimaru M, Castellanous EJ, Kaneda Y, Nakashima Y et al. Sp1 decoy transfected to carcinoma cells suppresses the expression of vascular endothelial growth factor, transforming growth factor beta1, and tissue factor and also cell growth and invasion activities. Cancer Res 2000; 60: 6531–6536.
Liang F, Schaufele F, Gardner DG . Sp1 dependence of natriuretic peptide receptor A gene transcription in rat aortic smooth muscle cells. Endocrinology 1999; 140: 1695–1701.
Motojima M, Ando T, Yoshioka T . Sp1-like activity mediates angiotensin-II-induced plasminogen-activator inhibitor type-1 (PAI-1) gene expression in mesangial cells. Biochem J 2000; 349: 435–441.
Verrecchia F, Rossert J, Mauviel A . Blocking sp1 transcription factor broadly inhibits extracellular matrix gene expression in vitro and in vivo: implications for the treatment of tissue fibrosis. J Invest Dermatol 2001; 116: 755–763.
Yu JH, Schwartzbauer G, Kazlman A, Menon RK . Role of the Sp family of transcription factors in the ontogeny of growth hormone receptor gene expression. J Biol Chem 1999; 274: 34327–34336.
Yamashita J, Yoshimasa T, Arai H, Hiraoka J, Takaya K, Miyamoto Y et al. Identification of cis-elements of the human endothelin-A receptor gene and inhibition of the gene expression by the decoy strategy. J Biol Chem 1998; 273: 15993–15999.
Kawauchi M, Suzuki J, Morishita R, Wada Y, Izawa A, Tomita N et al. Gene therapy for attenuating cardiac allograft arteriopathy using ex vivo E2F decoy transfection by HVJ-AVE-liposome method in mice and nonhuman primates. Circ Res 2000; 87: 1063–1068.
Yamashita J, Yoshimasa T, Arai H, Hiraoka J, Takaya K, Miyamoto Y et al. Identification of cis-elements of the human endothelin-A receptor gene and inhibition of the gene expression by the decoy strategy. J Biol Chem 1998; 273: 15993–15999.
Ahn JD, Morishita R, Kaneda Y, Lee SJ, Kwon KY, Choi SY et al. Inhibitory effects of novel AP-1 decoy oligodeoxynucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res 2002; 90: 1325–1332.
Ahn JD, Kim CH, Magae J, Kim YH, Kim HJ, Park KK et al. E2F decoy oligodeoxynucleotides effectively inhibit growth of human tumor cells. Biochem Biophys Res Commun 2003; 310: 1048–1053.
Chae YM, Park KK, Magae J, Lee IS, Kim CH, Kim HC et al. Sp1-decoy oligodeoxynucleotide inhibits high glucose-induced mesangial cell proliferation. Biochem Biophys Res Commun 2004; 319: 550–555.
Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 2002; 6: 219–226.
Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q et al. Transcriptional activation of transforming growth factor β1 and its receptors by the kruppel-like Factor Zf9/core rromoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem 1998; 273: 33750–33758.
Poncelet AC, Schnaper HW . Sp1 and smad proteins cooperate to mediate transforming growth factor-β1-induced alpha2(I) collagen expression in human glomerular mesangial cells. J Biol Chem 2001; 276: 6983–6992.
Datta PK, Blake MC, Moses HL . Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-β-induced physical and functional interactions between smads and Sp1. J Biol Chem 2000; 275: 40014–40019.
Slansky JE, Li Y, Kaelin WG, Farnham PJ . A protein synthesis-dependent increase in E2F1 mRNA correlates with growth regulation of the dihydrofolate reductase promoter. Mol Cell Biol 1993; 13: 1610–1618.
Ahn JD, Morishita R, Kaneda Y, Kim HJ, Kim YD, Lee HJ et al. Transcription factor decoy for AP-1 reduces mesangial cell proliferation and extracellular matrix production in vitro and in vivo. Gene Therapy 2004; 11: 916–923.
Maruyama H, Higuchi N, Nishikawa Y, Hirahara H, Iino N, Kameda S et al. Kidney-targeted naked DNA transfer by retrograde renal vein injection in rats. Hum Gene Ther 2002; 13: 455–468.
Acknowledgements
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the MRC for Cardiovascular Diseases and Natural products, Dongguk University (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chae, YM., Park, KK., Lee, IK. et al. Ring-Sp1 decoy oligonucleotide effectively suppresses extracellular matrix gene expression and fibrosis of rat kidney induced by unilateral ureteral obstruction. Gene Ther 13, 430–439 (2006). https://doi.org/10.1038/sj.gt.3302696
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302696
Keywords
This article is cited by
-
Caveolin-1 regulation of Sp1 controls production of the antifibrotic protein follistatin in kidney mesangial cells
Cell Communication and Signaling (2019)
-
Transcription factor decoy against stem cells master regulators, Nanog and Oct-4: a possible approach for differentiation therapy
Tumor Biology (2015)
-
The inhibitory effect of chimeric decoy oligodeoxynucleotide against NF-κB and Sp1 in renal interstitial fibrosis
Journal of Molecular Medicine (2013)
-
Antisense Makes Sense in Engineered Regenerative Medicine
Pharmaceutical Research (2009)
-
N-methyl amine-substituted fluoxetine derivatives: New dopamine transporter inhibitors
Archives of Pharmacal Research (2009)