Abstract
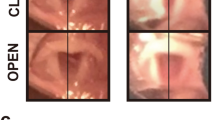
To assess the possibility of gene therapy for recurrent laryngeal nerve (RLN) injury, we examined functional and histological recovery after glial cell line-derived neurotrophic factor (GDNF) gene transfer in a rat RLN crush model. Adenoviral vector encoding β-galactosidase gene (AxCALacZ) or human GDNF gene (AxCAhGDNF) was injected into the crush site of the RLN. Neurons in the nucleus ambiguus on the crushed side were labeled with X-gal or GDNF immnohistochemistry after AxCALacZ or AxCAhGDNF injection. Reverse transcription-polymerase chain reaction analysis revealed expression of human GDNF mRNA transcripts in brainstem tissue containing the nucleus ambiguus on the crushed side after AxCAhGDNF injection. Animals injected with AxCAhGDNF displayed significantly improved motor nerve conduction velocity of the RLN and recovery rate of vocal fold movement at 2 and 4 weeks after treatment as compared to controls. AxCAhGDNF-injected animals showed a significantly larger axonal diameter and improved remyelination in crushed RLN as compared to controls. Adenoviral GDNF gene transfer may thus promote laryngeal function recovery after RLN injury. Inoculation of adenoviral vector containing the GDNF gene at the site of damage soon after nerve injury may assist patients with laryngeal paralysis caused by nerve injury during head and neck surgery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Isshiki N . Progress in laryngeal framework surgery. Acta Otolaryngol 2000; 120: 120–127.
Eisele DW . Complications of thyroid surgery. In: Eisele DW (ed) Complications in Head Neck Surgery. Mosby-Year Book: St Louis, 2000, pp 423–437.
Netterville JL, Aly A, Ossoff RH . Evaluation and treatment of complications of thyroid and parathyroid surgery. Otolaryngol Clin North Am 1990; 23: 529–552.
Goding GS . Nerve-muscle pedicle reinnervation of the paralyzed vocal cord. Otolaryngol Clin North Am 1991; 24: 1239–1252.
Horn KL, Crumley RL . The physiology of nerve injury and repair. Otolaryngo Clin North Am 1984; 17: 321–333.
Jacobs IN, Sanders I, Wu BL, Biller HF . Reinnervation of the canine posterior cricoarytenoid muscle with sympathetic preganglionic neurons. Ann Otol Rhinol Laryngol 1990; 99: 167–174.
Crumley RL . Mechanisms of synkinesis. Laryngoscope 1979; 89: 1847–1854.
Flint PW, Downs DH, Coltrera MD . Laryngeal synkinesis following reinnervation in the rat: neuroanatomic and physiologic study using retrograde fluorescent tracers and electromyography. Ann Otol Rhinol Laryngol 1991; 100: 797–806.
Singleton JR, Dixit VM, Feldman EL . Type I insulin-like growth factor receptor activation regulates apoptotic proteins. J Biol Chem 1996; 271: 31791–31794.
Kim B, Leventhal PS, Saltiel AR, Feldman EL . Insulin-like growth factor-I-mediated neurite outgrowth in vitro requires MAP kinase activation. J Biol Chem 1997; 272: 21268–21273.
Russell JW, Windebank AJ, Schenone A, Feldman EL . Insulin like growth factor-I prevents apoptosis in neurons after nerve growth factor withdrawal. J Neurobiol 1998; 36: 455–467.
Baumgartner BJ, Shine HD . Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci 1997; 17: 6504–6511.
Ribotta MG, Revah F, Pradier L, Loquet I, Mallet J, Privat A . Prevention of motor neuron death by adenovirus-mediated neurotrophic factors. J Neurosci Res 1997; 48: 281–285.
Lewin GR . Neurotrophic factors and pain. Semin Neurosci 1995; 7: 227–232.
Dittrich FH, Thoenen H, Sendtner M . Ciliary neurotrophic factor: pharmacokinetics and acute-phase response in rat. Ann Neurol 1994; 35: 151–163.
Houenou LJ, Oppenheim RW, Li L, Lo AC, Prevette D . Regulation of spinal motoneuron survival by GDNF during development and following injury. Cell Tissue Res 1996; 286: 219–223.
Lapchak PA, Jiao S, Miller PJ, Williams LR, Cummins V, Inouye G et al. Pharmacological characterization of GDNF as a therapeutic molecule for treating neurodegenerative diseases. Cell Tissue Res 1996; 286: 179–189.
Lindsay RM . Neuron saving schemes. Nature 1995; 373: 289–290.
Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M et al. GDNF: a potent survival factor for motoneurons present in peripheral. Science 1994; 266: 1062–1064.
Li L, Wu W, Lin LF, Lei M, Oppenheim RW, Houenou LJ . Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc Natl Acad Sci USA 1995; 92: 9771–9775.
Yan Q, Matheson C, Lopez OT . In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature 1995; 373: 341–344.
Sakamoto T, Watabe K, Ohashi T, Kawazoe Y, Oyanagi K, Inoue K et al. Adenoviral vector-mediated GDNF gene transfer prevents death of adult facial motoneurons. Neuroreport 2000; 11: 1857–1860.
Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW . Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science 1998; 279: 1725–1729.
Hoke A, Cheng C, Zochodne DW . Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport 2000; 11: 1651–1654.
Kang UJ . Genetic modification of cells with retrovirus vectors for grafting into the central nervous system. In: Kaplitt MG, Loewy AD (ed) Viral Vectors: Gene Therapy and Neuroscience Applications. Academic Press: San Diego, 1995, pp 211–237.
O’Malley Jr BW, Ledley FD . Somatic gene therapy in otolaryngology – head and neck surgery. Arch Otolaryngol Head Neck Surg 1993; 119: 1191–1197.
O’Malley Jr BW, Ledley FD . Somatic gene therapy: methods for the present and future. Arch Otolaryngol Head Neck surg 1993; 119: 1100–1177.
Shiotani A, O’Malley Jr BW, Coleman ME, Alila HW, Flint PW . Reinnervation of motor endplates and increased muscle fiber size after human insulin-like growth factor I gene transfer into the paralyzed larynx. Hum Gene Ther 1998; 9: 2039–2047.
Shiotani A, O’Malley Jr BW, Coleman ME, Flint PW . Human insulin-like growth factor 1 gene transfer into paralyzed rat larynx: single vs multiple injection. Arch Otolaryngol Head Neck Surg 1999; 125: 555–560.
Saito K, Shiotani A, Watabe K, Moro K, Fukuda H, Ogawa K . Adenoviral GDNF gene transfer prevents motoneuron loss in the nucleus ambiguus. Brain Res 2003; 962: 61–67.
Boulis NM, Bhatia V, Brindle TI, Holman HT, Krauss DJ, Blaivas M et al. Adenoviral nerve growth factor and beta-galactosidase transfer to spinal cord: a behavioral and histological analysis. J Neurosurg 1998; 90: 99–108.
Liu Y, Himes BT, Moul J, Huang W, Chow SY, Tessler A et al. Application of recombinant adenovirus for in vivo gene delivery to spinal cord. Brain Res 1997; 768: 19–29.
Boulis NM, Turner DE, Dice JA, Bhatia V, Feldman EL . Characterization of adenoviral gene expression in spinal cord after remote vector delivery. Neurosurgery 1999; 45: 131–138.
Ghadge GD, Roos RP, Kang UJ, Wollmann R, Fishman PS, Kalynych AM et al. CNS gene delivery by retrograde transport of recombinant replication – defective adenoviruses. Gene Therapy 1995; 2: 132–137.
Rubin AD, Hogikyan ND, Sullivan K, Boulis N, Feldman EL . Remote delivery of rAAV-GFP to the rat brainstem through the recurrent laryngeal nerve. Laryngoscope 2001; 111: 2041–2045.
Rubin A, Mobley B, Hogikyan N, Bell K, Sullivan K, Boulis N et al. Delivery of an adenoviral vector to the crushed recurrent laryngeal nerve. Laryngoscope 2003; 113: 985–989.
Varejao AS, Cabrita AM, Meek MF, Bulas-Cruz J, Melo-Pinto P, Raimondo S et al. Functional and morphological assessment of a standardized rat siatic nerve crush injury with a non-serrated clump. J Neurotrauma 2004; 21: 1652–1670.
Bridge P, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA et al. Nerve crush injuries – A model for axonotmesis. Exp Neurol 1994; 127: 284–290.
Seddon HJ . Three types of nerve injury. Brain 1943; 66: 237.
Sunderland S . Classification of peripheral nerve injury producing loss of function. Brain 1951; 74: 491.
Zeng L, Worseg A, Albrecht G, Grisold W, Hopf R, Redl H et al. A noninvasive functional evaluation following peripheral nerve repair with electromyography in a rat model. Plast Reconstr Surg 1994; 94: 146–151.
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L . Acute inflammatory response in spinal cord following impact injury. Exp Neurol 1998; 151: 77–88.
George JA . Gene therapy progress and prospects: adenoviral vectors. Gene Therapy 2003; 10: 1135–1141.
Check E . Sanctions agreed over teenager's gene-therapy death. Nature 2005; 433: 674.
Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003; 80: 148–158.
Watabe K, Ohashi T, Sakamoto T, Kawazoe Y, Takeshima T, Oyanagi K et al. Rescue of lesioned adult rat spinal motoneurons by adenoviral gene transfer of glial cell line-derived neurotrophic factor. J Neurosci Res 2000; 60: 511–519.
Kanegae Y, Takamori K, Sato Y, Lee G, Nakai M, Saito I . Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucl Acid Res 1995; 23: 3816–3821.
Sakamoto T, Kawazoe Y, Shen JS, Takeda Y, Arakawa Y, Ogawa J et al. Adenoviral gene transfer of GDNF, BDNF and TGFβ2, but not CNTF, cardiotrophin-1 or IGF1, protects injured adult motoneurons after facial nerve avulsion. J Neurosci Res 2003; 72: 54–64.
Acknowledgements
This work was supported in part by a Grant-in-aid for Scientific Research for Encouragement of Young Scientists (B) (No.14770921), a Grant-in-Aid for Scientific Research (C) (No. 15591831) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a National Grant-in-Aid for the Establishment of High-Tech Research Center in a Private University. A summary of this work was presented in a Research Forum at the 2004 Annual Meeting of the American Academy of Otolaryngology-Head and Neck Surgery in New York, NY, September 19–22, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araki, K., Shiotani, A., Watabe, K. et al. Adenoviral GDNF gene transfer enhances neurofunctional recovery after recurrent laryngeal nerve injury. Gene Ther 13, 296–303 (2006). https://doi.org/10.1038/sj.gt.3302665
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302665
Keywords
This article is cited by
-
An optimized method for high-quality RNA extraction from distinctive intrinsic laryngeal muscles in the rat model
Scientific Reports (2022)
-
TrkA inhibitor promotes motor functional regeneration of recurrent laryngeal nerve by suppression of sensory nerve regeneration
Scientific Reports (2020)
-
Hepatocyte Growth Factor (HGF) Promotes Peripheral Nerve Regeneration by Activating Repair Schwann Cells
Scientific Reports (2018)
-
Functional regeneration of the transected recurrent laryngeal nerve using a collagen scaffold loaded with laminin and laminin-binding BDNF and GDNF
Scientific Reports (2016)
-
Neuroprotection using gene therapy to induce vascular endothelial growth factor-A expression
Gene Therapy (2009)