Abstract
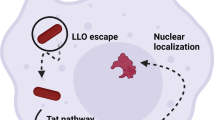
Recent advances in gene therapy can be attributed to improvements of gene delivery vectors. New viral and nonviral transport vehicles that considerably increase the efficiency of transfection have been prepared. However, these vectors still have many disadvantages that are difficult to overcome, thus, a new approach is needed. The approach of bacterial delivery could in the future be important for gene therapy applications. In this article we try to summarize the most important modifications that are used for the preparation of applied strains, difficulties that are related with bacterial gene delivery and the current use of bactofection in animal experiments and clinical trials. Important differences to the alternative gene therapy (AGT) are discussed. AGT resembles bacteria-mediated protein delivery, as the therapeutical proteins are produced not by host cells but by the bacteria in situ and the expression can be regulated exogenously. Although the procedure of bacterial gene delivery is far from being definitely solved, bactofection remains a promising technique for transfection in human gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mountain A . Gene therapy: the first decade. Trends Biotechnol 2000; 18: 119–128.
Hacein-Bey-Abina S et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003; 302: 415–419.
Gardlik R et al. Vectors and delivery systems in gene therapy. Med Sci Monit 2005; 11: RA110–RA121.
Page DT, Cudmore S . Innovations in oral gene delivery: challenges and potentials. Drug Discov Today 2001; 6: 92–101.
Loessner H, Weiss S . Bacteria-mediated DNA transfer in gene therapy and vaccination. Expert Opin Biol Ther 2004; 4: 157–168.
Schaffner W . Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci USA 1980; 77: 2163–2167.
Weiss S, Chakraborty T . Transfer of eukaryotic expression plasmids to mammalian host cells by bacterial carriers. Curr Opin Biotechnol 2001; 12: 467–472.
Fu GF et al. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther 2005; 12: 133–140.
Urashima M et al. An oral CD40 ligand gene therapy against lymphoma using attenuated Salmonella typhimurium. Blood 2000; 95: 1258–1263.
Darji A et al. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol Med Microbiol 2000; 27: 341–349.
Michael A et al. Novel strains of Salmonella typhimurium as potential vectors for gene delivery. FEMS Microbiol Letts 2004; 238: 345–351.
Shen H et al. Modulation of the immune system by Listeria monocytogenes-mediated gene transfer into mammalian cells. Microbiol Immunol 2004; 48: 329–337.
Celec P et al. The use of transformed Escherichia coli for experimental angiogenesis induced by regulated in situ production of vascular endothelial growth factor - an alternative gene therapy. Med Hypotheses 2005; 64: 505–511.
Fajac I et al. Recombinant Escherichia coli as a gene delivery vector into airway epithelial cells. J Control Release 2004; 97: 371–381.
Grillot-Courvalin C et al. Functional gene transfer from intracellular bacteria to mammalian cells. Nat Biotechnol 1998; 16: 862–866.
Pilgrim S et al. Bactofection of mammalian cells by Listeria monocytogenes: improvement and mechanism of DNA delivery. Gene Therapy 2003; 10: 2036–2045.
Dietrich G et al. Delivery of antigen-encoding plasmid DNA into the cytosol of macrophages by attenuated suicide Listeria monocytogenes. Nat Biotechnol 1998; 16: 181–185.
Grillot-Courvalin C, Goussard S, Courvalin P . Bacteria as gene delivery vectors for mammalian cells. Curr Opin Biotechnol 1999; 10: 477–481.
Weiss S . Transfer of eukaryotic expression plasmids to mammalian hosts by attenuated Salmonella spp. Int J Med Microbiol 2003; 293: 95–106.
Nemunaitis J et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther 2003; 10: 737–744.
Sznol M et al. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest 2000; 105: 1027–1030.
Zhao M et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102: 755–760.
Toso JF et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20: 142–152.
Medina E, Guzman CA . Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 2001; 19: 1573–1580.
Fox ME et al. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Therapy 1996; 3: 173–178.
Chakrabarty AM . Microorganisms and cancer: quest for a therapy. J Bacteriol 2003; 185: 2683–2686.
Liu SC et al. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Therapy 2002; 9: 291–296.
Minton NP . Clostridia in cancer therapy. Nat Rev Microbiol 2003; 1: 237–242.
Niethammer AG et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 2002; 8: 1369–1375.
Lee CH, Wu CL, Shiau AL . Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther 2005; 12: 175–184.
Krusch S et al. Listeria monocytogenes mediated CFTR transgene transfer to mammalian cells. J Gene Med 2002; 4: 655–667.
Darji A et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 1997; 91: 765–775.
Dietrich G et al. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr Opin Mol Ther 2003; 5: 10–19.
Timmons L, Fire A . Specific interference by ingested dsRNA. Nature 1998; 395: 854.
Timmons L, Court DL, Fire A . Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 2001; 263: 103–112.
Theys J et al. Stable Escherichia coli-Clostridium acetobutylicum shuttle vector for secretion of murine tumor necrosis factor alpha. Appl Environ Microbiol 1999; 65: 4295–4300.
Klink D et al. Gene delivery systems--gene therapy vectors for cystic fibrosis. J Cyst Fibros 2004; 3 (Suppl 2): 203–212.
Paglia P et al. In vivo correction of genetic defects of monocyte/macrophages using attenuated Salmonella as oral vectors for targeted gene delivery. Gene Therapy 2000; 7: 1725–1730.
Li X et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther 2003; 10: 105–111.
Saltzman DA et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm 1996; 11: 145–153.
Acknowledgements
We are greatful to L'ubomír Tomáška for the motivation to write this review. The authors are supported by Grant of the Comenius University 116/2004 and 117/2004 and the Grant Agency for Science and Technology APVT-20-003104.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pálffy, R., Gardlík, R., Hodosy, J. et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther 13, 101–105 (2006). https://doi.org/10.1038/sj.gt.3302635
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302635
Keywords
This article is cited by
-
Genetically engineered E. coli invade epithelial cells and transfer their genetic cargo into the cells: an approach to a gene delivery system
Biotechnology Letters (2023)
-
Genetic interference exerted by Salmonella-delivered CRISPR/Cas9 significantly reduces the pathological burden caused by Marek’s disease virus in chickens
Veterinary Research (2021)
-
Recent trends and advances in microbe-based drug delivery systems
DARU Journal of Pharmaceutical Sciences (2019)
-
In vivo reprogramming in inflammatory bowel disease
Gene Therapy (2013)