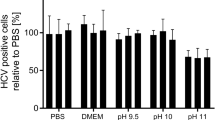
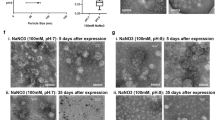
Abstract
Viral vectors and viral vaccines more and more play an important role in current medical approaches. Gene vectors like adenoviruses, adeno-associated viruses or retroviruses are the vehicles being developed for delivering genetic material to the target cell in gene therapy. Viral vaccines, like attenuated or inactivated rabies virus, influenza virus or hepatitis virus vaccines, are powerful tools to limit the number of serious viral infections and pandemics. Higher safety demands, that is, reduction of side effects, by regulatory authorities like Food and Drug Administration (FDA) and European Agency for the Evaluation of Medicinal Products (EMEA), nowadays force developers as well as manufacturers to improve their production and purification processes for viral vectors and vaccines. Like for influenza viral vaccines, manufacturers begin to switch from egg cultivation to mammalian cell culture systems. Also within the purification procedure, a clear trend from classical purification methods like sucrose gradient centrifugation towards more sophisticated techniques like tangential flow filtration and liquid chromatography can be observed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blaese R et al. T lymphocyte-directed gene therapy for ADA deficiency SCID: initial trial results after 4 years. Science 1995; 270: 475–480.
The Journal of Gene Medicine, Gene Therapy Clinical Trials, Charts and Tables, Vectors, 2005; http://82.182.180.141/trials/FMPro?-db=Trials.FP5&-format=vectors.html&-lay=Vectors&-findAll.
Lyddiatt A, O'Sullivan D . Biochemical recovery and purification of gene therapy vectors. Curr Opin Biotechnol 1998; 9: 177–185.
Wu N, Ataai M . Production of viral vectors for gene therapy applications. Curr Opin Biotechnol 2000; 11: 205–208.
Braas G, Searle P, Slater N, Lyddiatt A . Strategies for the isolation and purification of retroviral vectors for gene therapy. Bioseparation 1996; 6: 211–228.
Guilherme N, Monteiro G, Prozeres D, Cabral J . Downstream processing of plasmid DNA for gene therapy and DNA vaccine applications. TIBTECH 2000; 18: 380–388.
Zolotukhin S et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Therapy 1999; 6: 973–985.
Saha K, Lin Y-C, Wong P . A single method for obtaining highly viable virus from culture supernatant. J Virol Methods 1994; 46: 349–352.
Aboud M, Wolfson M, Hassan Y, Huleihel M . Rapid purification of extracellular and intracellular Moloney murine leukemia virus. Arch Virol 1982; 71: 185–195.
Morling F, Russell S . Enhanced transduction efficiency of retroviral vectors coprecipitated with calcium phosphate. Gene Therapy 1995; 2: 504–508.
Andreadis S et al. Large-scale processing of recombinant retroviruses for gene therapy. Biotechnol Prog 1999; 15: 1–11.
Hammar L, Gilljam G . Extraction of HIV-1 in aqueous two-phase systems to obtain a high yield of gp120. AIDS Res Hum Retroviruses 1990; 6: 1379–1388.
Morenweiser R . Amersham Biosciences. Downstream 2004; 37: 20–21.
McGrath M, Witte O, Pincus T, Weissman I . Retrovirus purification: method that conserves envelope glycoprotein and maximizes infectivity. J Virol 1978; 25: 923–927.
Ljunglof A, Bergvall P, Bhikhabhai R, Hjorth R . Direct visualization of plasmid DNA in individual chromatography adsorbent particles by confocal scanning laser microscopy. J Chromatogr A 1999; 844: 129–135.
Martin M, El Azher M, Vasseur M . Ionic strength- and temperature-induced KCa shifts in the uncoating reaction of rotavirus strains RF and SA11: correlation with membrane permeabilization. J Virol 2002; 76: 552–559.
Specht R et al. Densonucleosis virus purification by ion exchange membranes. Biotechnol Bioeng 2004; 88: 465–473.
Huyghe B et al. Purification of a type 5 recombinant adenovirus encoding human p53 by column chromatography. Hum Gene Ther 1995; 6: 1403–1416.
Herzer S, Wegmann T, Beckett P . Isoelectric titration curves of viral particles as an evaluation tool for ion exchange chromatography. Life Sci News 2003; 13: 16–18.
Valdes R et al. Hepatitis B surface antigen immunopurification using a plant-derived specific antibody produced in large scale. Biochim Biophys Res Commun 2003; 310: 742–747.
Grimm D, Kern A, Rittner K, Kleinschmidt J . Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther 1998; 9: 2745–2760.
Walther W, Stein U . Viral vectors for gene transfer. Drugs 2000; 60: 249–271.
Higashikawa F, Chang L . Kinetic analyses of stability of simple and complex retroviral vectors. Virology 2001; 280: 124–133.
Darling D et al. Low-speed centrifugation of retroviral vectors absorbed to a particulate substrate: a highly effective means of enhancing retroviral titer. Gene Therapy 2000; 7: 914–923.
Kuiper M, Sanches R, Walford J, Slater N . Purification of a functional gene therapy vector derived from Moloney murine leukemia virus using membrane filtration and ceramic hydroxyapatite chromatography. Biotechnol Bioeng 2002; 80: 445–453.
Baekelandt V et al. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Therapy 2003; 10: 1933–1940.
Zhang B et al. A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Therapy 2001; 8: 1745–1751.
Reiser J . Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Therapy 2000; 7: 910–913.
Richieri S et al. Characterization of highly purified, inactivated HIV-1 particles isolated by anion exchange chromatography. Vaccine 1998; 16: 119–129.
Bondoc L, Fitzpatrick S . Size distribution analysis of recombinant adenovirus using disc centrifugation. J Ind Microbiol Biotechnol 1998; 20: 317–322.
Green A et al. A new scalable method for the purification of recombinant adenovirus vectors. Hum Gene Ther 2002; 3: 1921–1934.
Kamen A, Henry O . Development and optimization of an adenovirus production process. J Gene Med 2004; 6: 184–192.
Smith A . Viral vectors in gene therapy. Annu Rev Microbiol 1995; 49: 807–838.
Blanche F et al. An improved anion-exchange HPLC method for the detection and purification of adenoviral particles. Gene Therapy 2000; 7: 1055–1062.
Smith R, Ding C, Kotin R . Serum-free production and column purification of adeno-associated virus type 5. J Virol Meth 2003; 114: 115–124.
Davidoff A et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J Virol Methods 2004; 121: 209–215.
O'Riordan C, Lachapelle A, Vincent K, Wadsworth S . Scaleable chromatographic purification process for recombinant adeno-associated virus (rAAV). Gene Med 2000; 2: 444–454.
Summerford C, Samulski R . Viral receptors and vector purification: new approaches for generating clinical-grade reagents. Nat Med 1999; 5: 587–588.
Summerford C, Samulski R . Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 vectors. J Virol 1998; 72: 1438–1445.
Auricchio A, O'Connor E, Hildinger M, Wilson J . A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol Ther 2001; 4: 372–374.
Clark K, Liu M, McGrath J, Johnson P . Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild type viruses. Hum Gene Ther 1999; 10: 1031–1039.
Brument N et al. A versatile and scalable two-step ion-exchange chromatography process for the purification of recombinant adeno-associated virus serotypes-2 and -5. Mol Ther 2002; 6: 678–686.
Blouin V et al. Improving rAAV production and purification: towards the definition of a scaleable process. J Gene Med 2004; 6: 223–228.
Siegel J . Gene Therapy: Is There Oversight for Patient Safety?. Testimony before the Senate Committee on Health, Education, Labor and Pensions, Subcommittee on Public Health, February 2, 2000.
The European Agency for the Evaluation of Medicinal Products, Note for Guidance on Quality, Preclinical and clinical aspects of gene transfer medicinal products, 9CPMP/BWP/3088/99, London, April 24, 2001, pp 1–33, http://www.emea.eu.int/pdfs/human/bwp/308899en.pdf.
Food and Drug Administration [Docket No. 2004D–0465], Draft Guidance for Food and Drug Administration Review Staff and Sponsors: Content and Review of Chemistry, Manufacturing, and Control Information for Human Gene Therapy Investigational New Drug Applications, November 1, 2004, pp 1–4, http://www.fda.gov/ohrms/dockets/98fr/cb0240.pdf.
Food and Drug Administration Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy, dated March 1998, http://www.fda.gov/cber/gdlns/somgene.pdf.
Food and Drug Administration Guidance for Industry: Content and Format of Investigational New Drug Applications (INDs) for Phase 1 Studies of Drugs, Including Well-Characterized, Therapeutic, Biotechnology-derived Products, dated November 1995, http://www.fda.gov/cder/guidance/phase1.pdf.
Food and Drug Administration Draft Guidance for Reviewers: Instructions and Template for Chemistry, Manufacturing, and Control (CMC) Reviewers of Human Somatic Cell Therapy Investigational New Drug Applications (INDs), dated August 2003, http://www.fda.gov/cber/gdlns/cmcsomcell.pdf.
Borellini F, Ostrove J . The transfer of technology from the laboratory to the clinic: in-process controls and final product testing. In: Meager A (ed). Gene Therapy Technologies, Applications and Regulations. John Wiley & Sons Ltd: New York, 1999, pp 359–373.
Su Z-Z et al. Targeting gene expression selectively in cancer cells by using the progression-elevated gene-3 promoter. Proc Natl Acad Sci USA 2005; 102: 1059–1064.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morenweiser, R. Downstream processing of viral vectors and vaccines. Gene Ther 12 (Suppl 1), S103–S110 (2005). https://doi.org/10.1038/sj.gt.3302624
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302624
Keywords
This article is cited by
-
Poxvirus sensitivity of a novel diploid sheep embryonic heart cell line
Archives of Virology (2023)
-
Incubation Temperature and Period During Denarase Treatment and Microfiltration Affect the Yield of Recombinant Adenoviral Vectors During Downstream Processing
Molecular Biotechnology (2023)
-
Investigation of Kluyveromyces marxianus as a novel host for large‐scale production of porcine parvovirus virus‐like particles
Microbial Cell Factories (2021)
-
Production of HIV-1-based virus-like particles for vaccination: achievements and limits
Applied Microbiology and Biotechnology (2019)
-
Characterization and immunogenicity of norovirus capsid-derived virus-like particles purified by anion exchange chromatography
Archives of Virology (2013)