Abstract
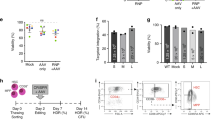
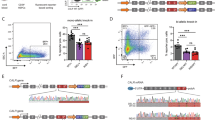
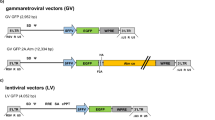
Wiskott–Aldrich syndrome (WAS) is an immune deficiency with thrombopenia resulting from mutations in the WASP gene. This gene normally encodes the Wiskott–Aldrich syndrome protein (WASP), a major cytoskeletal regulator expressed in hematopoietic cells. Gene therapy is a promising option for the treatment of WAS, requiring that clinically applicable WASP gene transfer vectors demonstrate efficacy in preclinical studies. Here, we describe a self-inactivating HIV-1-derived lentiviral vector encoding human WASP and show that it effectively transduced bone marrow progenitor cells of WASP knockout (WKO) mice. Transplantation of these transduced cells into lethally irradiated WKO recipients led to stable expression of WASP and correction of immune, inflammatory and cytoskeletal defects. Splenic T-cell proliferation was restored, podosomes were reinstated on bone-marrow-derived dendritic cells and colon inflammation was reduced. This shows for the first time (a) that cytoskeletal defects can be corrected in WKO mice, (b) that human WASP is biologically active in mice and (c) that a lentiviral vector is effective to express human WASP in vivo over several months. These data support further development of such lentiviral vectors for the gene therapy of WAS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thrasher AJ . WASp in immune-system organization and function. Nat Rev Immunol 2002; 2: 635–646.
Badour K, Zhang J, Siminovitch KA . The Wiskott–Aldrich syndrome protein: forging the link between actin and cell activation. Immunol Rev 2003; 192: 98–112.
Notarangelo LD, Ochs HD . Wiskott–Aldrich syndrome: a model for defective actin reorganization, cell trafficking and synapse formation. Curr Opin Immunol 2003; 15: 585–591.
Dupuis-Girod S et al. Autoimmunity in Wiskott–Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 2003; 111: e622–e627.
Imai K et al. Clinical course of patients with WASP gene mutations. Blood 2004; 103: 456–464.
Derry JM et al. WASP gene mutations in Wiskott–Aldrich syndrome and X-linked thrombocytopenia. Hum Mol Genet 1995; 4: 1127–1135.
Sasahara Y et al. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell 2002; 10: 1269–1281.
Dupre L et al. Wiskott–Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 2002; 17: 157–166.
Orange JS et al. Wiskott–Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA 2002; 99: 11351–11356.
Jones GE et al. Restoration of podosomes and chemotaxis in Wiskott–Aldrich syndrome macrophages following induced expression of WASp. Int J Biochem Cell Biol 2002; 34: 806–815.
Calle Y et al. WASp deficiency in mice results in failure to form osteoclast sealing zones and defects in bone resorption. Blood 2004; 103: 3552–3561.
Snapper SB et al. Wiskott–Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 1998; 9: 81–91.
Zhang J et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J Exp Med 1999; 190: 1329–1342.
Klein C et al. Gene therapy for Wiskott–Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood 2003; 101: 2159–2166.
Wada T, Jagadeesh GJ, Nelson DL, Candotti F . Retrovirus-mediated WASP gene transfer corrects Wiskott–Aldrich syndrome T-cell dysfunction. Hum Gene Ther 2002; 13: 1039–1046.
Strom TS et al. Functional correction of T cells derived from patients with the Wiskott–Aldrich syndrome (WAS) by transduction with an oncoretroviral vector encoding the WAS protein. Gene Therapy 2003; 10: 803–809.
Filipovich AH et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott–Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood 2001; 97: 1598–1603.
Candotti F et al. Retrovirus-mediated WASP gene transfer corrects defective actin polymerization in B cell lines from Wiskott–Aldrich syndrome patients carrying ‘null’ mutations. Gene Therapy 1999; 6: 1170–1174.
Strom TS et al. Defects in T-cell-mediated immunity to influenza virus in murine Wiskott–Aldrich syndrome are corrected by oncoretroviral vector-mediated gene transfer into repopulating hematopoietic cells. Blood 2003; 102: 3108–3116.
Follenzi A et al. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 2000; 25: 217–222.
Linder S, Aepfelbacher M . Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 2003; 13: 376–385.
Burns S et al. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 2001; 98: 1142–1149.
Linder S et al. Macrophages of patients with X-linked thrombocytopenia display an attenuated Wiskott–Aldrich syndrome phenotype. Immunol Cell Biol 2003; 81: 130–136.
Biffi A et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Invest 2004; 113: 1118–1129.
Piacibello W et al. Lentiviral gene transfer and ex vivo expansion of human primitive stem cells capable of primary, secondary, and tertiary multilineage repopulation in NOD/SCID mice. Nonobese diabetic/severe combined immunodeficient. Blood 2002; 100: 4391–4400.
Levasseur DN, Ryan TM, Pawlik KM, Townes TM . Correction of a mouse model of sickle cell disease: lentiviral/anti-sickling {beta}-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood 2003; 102: 4312–4319.
Hacein-Bey-Abina S et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2003; 348: 255–256.
Petrella A et al. A 5′ regulatory sequence containing two Ets motifs controls the expression of the Wiskott–Aldrich syndrome protein (WASP) gene in human hematopoietic cells. Blood 1998; 91: 4554–4560.
Hagemann TL, Kwan SP . The identification and characterization of two promoters and the complete genomic sequence for the WiskottvAldrich syndrome gene. Biochem Biophys Res Commun 1999; 256: 104–109.
Towers GJ et al. One step screening of retroviral producer clones by real time quantitative PCR. J Gene Med 1999; 1: 352–359.
Acknowledgements
We are grateful to Dr S Snapper, Harvard Medical School for the gift of WKO mice. We are thankful to our colleagues at Genethon, G Griffith for vector construction, B Gjata and I Adamski for help with histology and to Dr J Davoust for help with confocal microscopy. We would also like to acknowledge the dedicated care provided to the mice by the personnel of the Institut Gustave Roussy animal facility. This study was supported in part by equipment funds from ‘Génopole Recherche’, Evry, France and from the ‘Fondation pour la Recherche Médicale’, Paris, France.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Charrier, S., Stockholm, D., Seye, K. et al. A lentiviral vector encoding the human Wiskott–Aldrich syndrome protein corrects immune and cytoskeletal defects in WASP knockout mice. Gene Ther 12, 597–606 (2005). https://doi.org/10.1038/sj.gt.3302440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302440
Keywords
This article is cited by
-
Lentiviral standards to determine the sensitivity of assays that quantify lentiviral vector copy numbers and genomic insertion sites in cells
Gene Therapy (2022)
-
Design of a titering assay for lentiviral vectors utilizing direct extraction of DNA from transduced cells in microtiter plates
Molecular Therapy - Methods & Clinical Development (2016)
-
Lentivirus-meditated frataxin gene delivery reverses genome instability in Friedreich ataxia patient and mouse model fibroblasts
Gene Therapy (2016)
-
Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy
Molecular Therapy - Methods & Clinical Development (2015)
-
Gene transfer into hematopoietic stem cells as treatment for primary immunodeficiency diseases
International Journal of Hematology (2014)