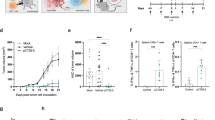
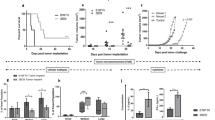
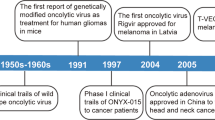
Abstract
The idea of enhancing or establishing effective immune response against endogenously developed tumor cells is not novel. More than a hundred years ago, bacterial components were used to develop antitumor immune response. Later, when a number of immune system-effecting cytokines had been discovered, they were used for systemic treatment of cancer patients. However, systemic treatment often resulted in even negative outcome. Recent developments of genetic approaches of cell modifications allowed developing of modern techniques of targeted tumor cell elimination. In the present paper, we review modern trends of the antitumor response enhancement based on immunoregulatory gene transfer into different cell types both in vivo and in vitro. Almost all these approaches are based on the activation of the adaptive arm of the immune system in response to tumor cells. However, recent studies indicate that the innate arm of the immune system, as well as adaptive arm, is involved in tumor suppression. The innate immune system uses nonrearranging germline receptors, which could trigger cellular effector responses that are conditional (or instructive) to the subsequent adaptive immune response. Last years' viewpoints on ‘self’ and ‘non-self’ recognition and primary induction of the immune response have changed. The key role of lymphocytes is pathogen recognition and, following immune response induction, switched on the central role of dendritic cells in ‘non-self’ recognition and induction of both innate and adaptive responses. Moreover, innate response is supposed to be an essential starting point in induction of successful and effective acquired response. Most cancer vaccines do not have ‘non-self’ marks presentation due to their endogenous origin, thus lacking their effectiveness in the induction of the specific long-lasting immune response. Taking this point into consideration, we can conclude that to make cancer vaccine more effective we have to present tumor antigens, together with the molecules that can potentially activate downstream ‘non-self’ recognition events not in parallel, but as a consequence of tumor antigen processing and presentation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Matzinger P . The danger model: a renewed sense of self. Science 2002; 296: 301–305.
Medzhitov R, Janeway Jr CA . Decoding the patterns of self and nonself by the innate immune system. Science 2002; 296: 298–300.
Aderem A, Ulevitch R . Toll-like receptors in the induction of the innate immune response. Nature 2000; 406: 782–787.
Schnare M et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol 2001; 2: 947–950.
Akira S, Takeda K, Kaisho T . Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2: 675–680.
Van Der Bruggen P et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev 2002; 188: 51–64.
Scanlan MJ et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 2002; 188: 22–32.
Emens LA, Jaffee EM . Cancer vaccines: an old idea comes of age. Cancer Biol Ther 2003; 2: S161–S168.
Ohashi PS et al. Ablation of ‘tolerance’ and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 1991; 65: 305–317.
Miller JF, Morahan G, Allison J . Extrathymic acquisition of tolerance by T lymphocytes. Cold Spring Harb Symp Quant Biol 1989; 54: 807–813.
Gallucci S, Matzinger P . Danger signals: SOS to the immune system. Curr Opin Immunol 2001; 13: 114–119.
Pardoll DM . Cancer vaccines. Nat Med 1998; 4: 525–531.
Khong HT, Restifo NP . Natural selection of tumor variants in the generation of ‘tumor escape’ phenotypes. Nat Immunol 2002; 3: 999–1005.
Lindenmann J, Klein PA . Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med 1967; 126: 93–108.
Fearon ER et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell 1990; 60: 397–403.
Gansbacher B et al. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med 1990; 172: 1217–1224.
Hock H et al. Mechanisms of rejection induced by tumor cell-targeted gene transfer of interleukin 2, interleukin 4, interleukin 7, tumor necrosis factor, or interferon gamma. Proc Natl Acad Sci USA 1993; 90: 2774–2778.
Tepper RI, Pattengale PK, Leder P . Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell 1989; 57: 503–512.
Golumbek PT et al. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 1991; 254: 713–716.
Schmidt-Wolf IG et al. Interleukin-7 gene transfer in patients with metastatic colon carcinoma, renal cell carcinoma, melanoma, or with lymphoma. Hum Gene Ther 1994; 5: 1161–1168.
Tahara H et al. Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector. J Immunol 1995; 154: 6466–6474.
Colombo MP et al. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med 1991; 173: 889–897.
Dranoff G et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA 1993; 90: 3539–3543.
Restifo NP et al. A nonimmunogenic sarcoma transduced with the cDNA for interferon gamma elicits CD8+ T cells against the wild-type tumor: correlation with antigen presentation capability. J Exp Med 1992; 175: 1423–1431.
Rosenberg SA . Progress in human tumour immunology and immunotherapy. Nature 2001; 411: 380–384.
Bubenik J et al. Local administration of cells containing an inserted IL-2 gene and producing IL-2 inhibits growth of human tumours in nu/nu mice. Immunol Lett 1988; 19: 279–282.
Zitvogel L et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol 1995; 55: 1393–1403.
Steinman RM, Cohn ZA . Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137: 1142–1162.
Timmerman JM, Levy R . Dendritic cell vaccines for cancer immunotherapy. Annu Rev Med 1999; 50: 507–529.
Boczkowski D, Nair SK, Snyder D . Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 1996; 184: 465–472.
Heiser A et al. Human dendritic cells transfected with RNA encoding prostate-specific antigen stimulate prostate-specific CTL responses in vitro. J Immunol 2000; 164: 5508–5514.
Morse MA et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest 2003; 21: 341–349.
Thornburg C et al. Induction of cytotoxic T lymphocytes with dendritic cells transfected with human papillomavirus E6 and E7 RNA: implications for cervical cancer immunotherapy. J Immunother 2000; 23: 412–418.
Nishioka Y et al. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res 1999; 59: 4035–4041.
Rosenberg SA, Spiess P, Lafreniere R . A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233: 1318–1321.
Arakawa F et al. Targeting of T cells to CEA-expressing tumor cells by chimeric immune receptors with a highly specific single-chain anti-CEA activity. Anticancer Res 2002; 22: 4285–4289.
Gritzapis AD, Mamalaki A, Kretsovali A . Redirecting mouse T hybridoma against human breast and ovarian carcinomas: in vivo activity against HER-2/neu expressing cancer cells. Br J Cancer 2003; 88: 1292–1300.
Trompeter HI et al. Rapid and highly efficient gene transfer into natural killer cells by nucleofection. J Immunol Methods 2003; 274: 245–256.
Schirrmann T, Pecher G . Human natural killer cell line modified with a chimeric immunoglobulin T-cell receptor gene leads to tumor growth inhibition in vivo. Cancer Gene Ther 2002; 9: 390–398.
Gussow D, Seemann G . Humanization of monoclonal antibodies. Methods Enzymol 1991; 203: 99–121.
Hudson PJ . Recombinant antibodies: a novel approach to cancer diagnosis and therapy. Expert Opin Investig Drugs 2000; 9: 1231–1242.
Perez N et al. Regulatable systemic production of monoclonal antibodies by in vivo muscle electroporation. Genet Vaccines Ther 2004; 2: 2.
Tjelle TE et al. Monoclonal antibodies produced by muscle after plasmid injection and electroporation. Mol Ther 2004; 9: 328–336.
Wolff JA et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247: 1465–1468.
Tang DC, DeVit M, Johnston SA . Genetic immunization is a simple method for eliciting an immune response. Nature 1992; 356: 152–154.
Krieg AM . CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002; 20: 709–760.
Wang B et al. Vaccination against pathogenic cells by DNA inoculation. Curr Top Microbiol Immunol 1998; 226: 21–35.
Donnelly JJ et al. DNA vaccines. Annu Rev Immunol 1997; 15: 617–648.
Irvine KR et al. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol 1996; 156: 238–245.
Diefenbach A, Raulet DH . The innate immune response to tumors and its role in the induction of T-cell immunity. Immunol Rev 2002; 188: 9–21.
Ruzek MC et al. Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol Ther 2002; 5: 115–124.
Rudginsky S et al. Antitumor activity of cationic lipid complexed with immunostimulatory DNA. Mol Ther 2001; 4: 347–355.
Ma Y et al. Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding protein dependent cell-mediated cytotoxicity. Proc Natl Acad Sci USA 1999; 96: 371–375.
Biragyn A et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol 2001; 167: 6644–6653.
Larin SS et al. Immunotherapy with autologous tumor cells engineered to secrete Tag7/PGRP, an innate immunity recognition molecule. J Gene Med 2004; 6: 798–808.
Fushimi T et al. Macrophage inflammatory protein 3alpha transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. J Clin Invest 2000; 105: 1383–1393.
Sfondrini L et al. Anti-tumor immunity induced by murine melanoma cells transduced with the Mycobacterium tuberculosis gene encoding the 38-kDa antigen. Gene Therapy 1998; 5: 247–252.
Mule JJ et al. RANTES secretion by gene-modified tumor cells results in loss of tumorigenicity in vivo: role of immune cell subpopulations. Hum Gene Ther 1996; 7: 1545–1553.
Pulaski BA, Ostrand-Rosenberg S . Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res 1998; 58: 1486–1493.
Fenton RT et al. A phase I trial of B7-transfected or parental lethally irradiated allogeneic melanoma cell lines to induce cell-mediated immunity against tumor-associated antigen presented by HLA-A2 or HLA-A1 in patients with stage IV melanoma. NCI protocol T93-0161. BRMP protocol 9401. Hum Gene Ther 1995; 6: 87–106.
Zitvogel L et al. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol 1996; 26: 1335–1341.
Nabel GJ et al. Immune response in human melanoma after transfer of an allogeneic class I major histocompatibility complex gene with DNA–liposome complexes. Proc Natl Acad Sci USA 1996; 93: 15388–15393.
Simons JW et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res 1997; 57: 1537–1546.
Kircheis R et al. Cytokine gene-modified tumor cells for prophylactic and therapeutic vaccination: IL-2, IFN-gamma, or combination IL-2+IFN-gamma. Cytokines Cell Mol Ther 1998; 4: 95–103.
Chong H et al. Tumour cell expression of B7 costimulatory molecules and interleukin-12 or granulocyte-macrophage colony-stimulating factor induces a local antitumour response and may generate systemic protective immunity. Gene Therapy 1998; 5: 223–232.
Parney IF et al. Granulocyte-macrophage colony-stimulating factor and B7-2 combination immunogene therapy in an allogeneic Hu-PBL-SCID/beige mouse-human glioblastoma multiforme model. Hum Gene Ther 1997; 8: 1073–1085.
Zitvogel L et al. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol 1996; 26: 1335–1341.
Pulaski BA et al. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res 2000; 60: 2710–2715.
Kiselev SL et al. Molecular cloning and characterization of the mouse tag7 gene encoding a novel cytokine. J Biol Chem 1998; 273: 18633–18639.
Kang D et al. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc Natl Acad Sci USA 1998; 95: 10078–10082.
Kibardin AV et al. Molecular cloning of a new mouse gene tagL containing a lysozyme-like domain. Dokl Biochem 2000; 372: 103–105.
Werner T et al. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA 2000; 97: 13772–13777.
Liu C et al. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem 2001; 276: 34686–34694.
Rehman A et al. The cloning of a rat peptidoglycan recognition protein (PGRP) and its induction in brain by sleep deprivation. Cytokine 2001; 13: 8–17.
Tydell CC et al. Isolation, characterization, and antimicrobial properties of bovine oligosaccharide binding protein: a microbicidal granule protein of eosinophils and neutrophils. J Biol Chem 2002; 277: 19658–19664.
Sashchenko LP et al. Peptidoglycan recognition protein tag7 forms a cytotoxic complex with heat shock protein 70 in solution and in lymphocytes. J Biol Chem 2004; 279: 2117–2124.
Lo D et al. Peptidoglycan recognition protein expression in mouse Peyer's Patch follicle associated epithelium suggests functional specialization. Cell Immunol 2003; 224: 8–16.
Mirkina II et al. Cloning and study of novel mammalian genes with structural homology with phage lysozyme. Genetika 2000; 36: 1492–1500.
Kibardin AV et al. The differentially spliced mouse tagL gene, homolog of tag7/PGRP gene family in mammals and Drosophila, can recognize Gram-positive and Gram-negative bacterial cell wall independently of T phage lysozyme homology domain. J Mol Biol 2003; 326: 467–474.
Yoshida H, Kinoshita K, Ashida M . Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J Biol Chem 1996; 271: 13854–13860.
Michel T et al. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 2001; 414: 756–759.
Gottar M et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002; 416: 640–644.
Werner T et al. Functional diversity of the Drosophila PGRP-LC gene cluster in the response to lipopolysaccharide and peptidoglycan. J Biol Chem 2003; 278: 26319–26322.
Choe KM et al. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002; 296: 359–362.
Ramet M et al. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002; 416: 644–648.
Moiseynko VM et al. Phase I/II trial of gene therapy with the autologous tumor cells modified with the tag7/PGRP-S gene in patients with the disseminated solid tumors. Ann Oncol 2004, (in press).
Acknowledgements
We thank Mauro Mezzina for helpful suggestions and for kind patience during all stages of manuscript preparation and reviewing. Studies by the authors that are reported here have been supported by grants from the Moscow Anticancer Program, Russian Foundation for Basic Research and Frontiers in Genetics.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larin, S., Georgiev, G. & Kiselev, S. Gene transfer approaches in cancer immunotherapy. Gene Ther 11 (Suppl 1), S18–S25 (2004). https://doi.org/10.1038/sj.gt.3302365
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302365
Keywords
This article is cited by
-
siRNA-mediated silencing of PD-1 ligands enhances tumor-specific human T-cell effector functions
Gene Therapy (2012)
-
Targets for active immunotherapy against pediatric solid tumors
Cancer Immunology, Immunotherapy (2009)
-
Nanosized bioceramic particles could function as efficient gene delivery vehicles with target specificity for the spleen
Gene Therapy (2007)
-
Gene therapy for carcinoma of the breast
Cancer Gene Therapy (2006)
-
Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model
Cancer Gene Therapy (2006)