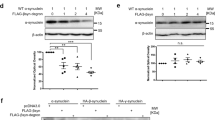
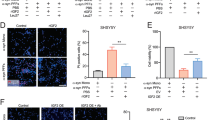
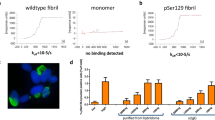
Abstract
Current experimental gene therapy approaches for Parkinson's disease (PD) and dementia with Lewy bodies (DLB) include the use of viral vectors expressing antiapoptosis genes, neurotrophic factors and dopaminergic system enzymes. However, since increasing evidence favors a role for α-synuclein accumulation in the pathogenesis of these disorders, an alternative therapy might require the transfer of genes that might block α-synuclein accumulation. β-Synuclein, the nonamyloidogenic homologue of α-synuclein, has recently been identified as a potential candidate. Thus, in vivo transfer of genes encoding β-synuclein might provide a novel approach to the development of experimental treatments for PD and DLB. To assess this possibility and to better understand the mechanisms involved, a lentiviral vector expressing human (h) β-synuclein (lenti-β-synuclein) was tested in a transgenic (tg) mouse model of hα-synuclein aggregation. This study showed that unilateral intracerebral injection of lenti-β-synuclein reduced the formation of hα-synuclein inclusions and the accumulation of hα-synuclein in synapses and ameliorated the neurodegenerative alterations in the tg mice. Both in vivo and in vitro coimmunoprecipitation and immunoblot experiments show that the mechanisms of β-synuclein neuroprotection involve binding of this molecule to hα-synuclein and Akt, resulting in the decreased aggregation and accumulation of hα-synuclein in the synaptic membrane. Together, these data further support a role for β-synuclein in regulating the conformational state of α-synuclein and suggest that this gene transfer approach might have potential for the development of alternative therapies for PD and DLB.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Burton EA, Glorioso JC, Fink DJ . Gene therapy progress and prospects: Parkinson's disease. Gene Therapy 2003; 10: 1721–1727.
Bjorklund A et al. Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res 2000; 886: 82–98.
Trojanowski J, Goedert M, Iwatsubo T, Lee V . Fatal attractions: abnormal protein aggregation and neuron death in Parkinson's disease and Lewy body dementia. Cell Death Differ 1998; 5: 832–837.
Hashimoto M, Masliah E . Alpha-synuclein in Lewy body disease and Alzheimer's disease. Brain Pathol 1999; 9: 707–720.
Koo E, Lansbury PJ, Kelly J . Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA 1999; 96: 9989–9990.
Conway KA et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA 2000; 97: 571–576.
Paik S et al. Self-oligomerization of NACP, the precursor protein of the non-amyloid beta/A4 protein (A beta) component of Alzheimer's disease amyloid, observed in the presence of a C-terminal A beta fragment (residues 25–35). FEBS Lett 1998; 421: 73–76.
Hashimoto M et al. Human recombinant NACP/a-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res 1998; 799: 301–306.
Volles MJ et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry 2001; 40: 7812–7819.
Narayanan V, Scarlata S . Membrane binding and self-association of alpha-synucleins. Biochemistry 2001; 40: 9927–9934.
Conway K, Harper J, Lansbury P . Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 1998; 4: 1318–1320.
Narhi L et al. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem 1999; 274: 9843–9846.
Osterova-Golts N et al. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 2000; 20: 6048–6054.
Kanda S et al. Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience 2000; 97: 279–284.
Cohen G . Oxidative stress, mitochondrial respiration, and Parkinson's disease. Ann NY Acad Sci 2000; 899: 112–120.
Masliah E, Hashimoto M . Development of new treatments for Parkinson's disease in transgenic animal models: a role for beta-synuclein. Neurotoxicology 2002; 23: 461–468.
Klucken J et al. Hsp70 reduces alpha-synuclein aggregation and toxicity. J Biol Chem 2004; 279: 25497–25502.
Clayton D, George J . The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. TINS 1998; 21: 249–254.
Maroteaux L, Scheller R . The rat brain synucleins; family of proteins transiently associated with neuronal membrane. Mol Brain Res 1991; 11: 335–343.
Levedan C . The synuclein family. Genome Res 1998; 8: 871–880.
Hashimoto M et al. b-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron 2001; 32: 213–223.
Giasson BI, Murray IV, Trojanowski JQ, Lee VM . A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem 2001; 276: 2380–2386.
Rockenstein E et al. Altered expression of the synuclein family mRNA in Lewy body and Alzheimer's disease. Brain Res 2001; 914: 48–56.
Blomer U et al. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 1997; 71: 6641–6649.
Englund U et al. The use of a recombinant lentiviral vector for ex vivo gene transfer into the rat CNS. NeuroReport 2000; 11: 3973–3977.
Kafri T, van Praag H, Gage FH, Verma IM . Lentiviral vectors: regulated gene expression. Mol Ther 2000; 1: 516–521.
Masliah E et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 2000; 287: 1265–1269.
Iwai A et al. The precursor protein of non-Ab component of Alzheimer's disease amyloid (NACP) is a presynaptic protein of the central nervous system. Neuron 1994; 14: 467–475.
Souza J, Giasson B, Lee V-Y, Ischiropoulos H . Chaperone-like activity of synucleins. FEBS Lett 2000; 474: 116–119.
Hashimoto M et al. beta-Synuclein regulates Akt activity in neuronal cells: a possible mechanism for neuroprotection in Parkinson's disease. J Biol Chem 2004; 279: 23622–23629.
Park JY, Lansbury Jr PT . Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson's disease. Biochemistry 2003; 42: 3696–3700.
Uversky VN et al. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem 2002; 277: 11970–11978.
Davidson W, Jonas A, Clayton D, George J . Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 1998; 273: 9443–9449.
Iwata A et al. alpha-Synuclein forms a complex with transcription factor Elk-1. J Neurochem 2001; 77: 239–252.
Lee HJ, Choi C, Lee SJ . Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem 2002; 277: 671–678.
Sharon R et al. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron 2003; 37: 583–595.
Lee HJ et al. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J Biol Chem 2002; 277: 5411–5417.
Perrin RJ, Woods WS, Clayton DF, George JM . Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem 2001; 276: 41958–41962.
Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI . The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 2002; 23: 843–853.
Datta SR, Brunet A, Greenberg ME . Cellular survival: a play in three Akts. Genes Dev 1999; 13: 2905–2927.
da Costa CA, Masliah E, Checler F . Beta-synuclein displays an antiapoptotic p53-dependent phenotype and protects neurons from 6-hydroxydopamine-induced caspase 3 activation: cross-talk with alpha-synuclein and implication for Parkinson's disease. J Biol Chem 2003; 278: 37330–37335.
Mayo LD, Donner DB . The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci 2002; 27: 462–467.
Georgievska B et al. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. NeuroReport 2002; 13: 75–82.
Takenouchi T et al. Reduced neuritic outgrowth and cell adhesion in neuronal cells transfected with human a-synuclein. Mol Cell Neurosci 2001; 17: 141–150.
Marr RA et al. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J Neurosci 2003; 23: 1992–1996.
Masliah E, Rockenstein E . Genetically altered transgenic models of Alzheimer's disease. J Neural Transm Suppl 2000; 59: 175–183.
Masliah E et al. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science 2000; 287: 1265–1269.
Rockenstein E et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res 2002; 68: 568–578.
Rockenstein E et al. Levels and alternative splicing of amyloid b protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. J Biol Chem 1995; 270: 28257–28267.
Mucke L et al. High-level neuronal expression of Abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 2000; 20: 4050–4058.
Acknowledgements
This work was supported by National Institutes of Health Grants AG5131, and AG18440 and by a grant from the MJ Fox Foundation for Parkinson's Research to EM and by AG08514 to FHG. RAM was supported in part by funds from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hashimoto, M., Rockenstein, E., Mante, M. et al. An antiaggregation gene therapy strategy for Lewy body disease utilizing β-synuclein lentivirus in a transgenic model. Gene Ther 11, 1713–1723 (2004). https://doi.org/10.1038/sj.gt.3302349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302349
Keywords
This article is cited by
-
Repurposing GLP-1 Receptor Agonists for Parkinson’s Disease: Current Evidence and Future Opportunities
Pharmaceutical Medicine (2021)
-
Elucidating Critical Proteinopathic Mechanisms and Potential Drug Targets in Neurodegeneration
Cellular and Molecular Neurobiology (2020)
-
Investigating the neuroprotective effect of AAV-mediated β-synuclein overexpression in a transgenic model of synucleinopathy
Scientific Reports (2018)
-
β-Synuclein suppresses both the initiation and amplification steps of α-synuclein aggregation via competitive binding to surfaces
Scientific Reports (2016)
-
Protection against neurodegenerative disease on Earth and in space
npj Microgravity (2016)