Abstract
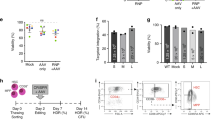
Erythropoietic protoporphyria (EPP) is an inherited defect of the ferrochelatase (FECH) gene characterized by the accumulation of toxic protoporphyrin in the liver and bone marrow resulting in severe skin photosensitivity. We previously described successful gene therapy of an animal model of the disease with erythroid-specific lentiviral vectors in the absence of preselection of corrected cells. However, the high-level of gene transfer obtained in mice is not translatable to large animal models and humans if there is no selective advantage for genetically modified hematopoietic stem cells (HSCs) in vivo. We used bicistronic SIN-lentiviral vectors coexpressing EGFP or FECH and the G156A-mutated O6-methylguanine-DNA-methyltransferase (MGMT) gene, which allowed efficient in vivo selection of transduced HSCs after O6-benzylguanine and BCNU treatment. We demonstrate for the first time that the correction and in vivo expansion of deficient transduced HSC population can be obtained by this dual gene therapy, resulting in a progressive increase of normal RBCs in EPP mice and a complete correction of skin photosensitivity. Finally, we developed a novel bipromoter SIN-lentiviral vector with a constitutive expression of MGMT gene to allow the selection of HSCs and with an erythroid-specific expression of the FECH therapeutic gene.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cavazzana-Calvo M et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000; 288: 669–672.
Hacein-Bey-Abina S et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002; 346: 1185–1193.
Schmidt M et al. Clonality analysis after retroviral-mediated gene transfer to CD34+ cells from the cord blood of ADA-deficient SCID neonates. Nat Med 2003; 9: 463–468.
Uchida N et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA 1998; 95: 11939–11944.
Miyoshi H et al. Efficient transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 1999; 283: 682–686.
SS Case et al. Stable transduction of quiescent CD34+CD38− human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci USA 1999; 96: 2988–2993.
Evans JR, Kelly PF, O’Neill E, Garcia JV . Human cord blood CD34+CD38− cell transduction via lentivirus-based gene transfer vectors. Hum Gene Ther 1999; 10: 1479–1489.
Sirven A et al. Enhanced transgene expression in cord blood CD34(+)-derived hematopoietic cells, including developing T cells and NOD/SCID mouse repopulating cells, following transduction with modified trip lentiviral vectors. Mol Ther 2001; 3: 438–448.
Miyoshi H et al. Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Zufferey R et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 1998; 72: 9873–9880.
Wu X et al. Development of a novel trans-lentiviral vector that affords predictable safety. Mol Ther 2000; 2: 47–55.
Logan AC, Lutzko C, Kohn DB . Advances in lentiviral vector design for gene-modification of hematopoietic stem cells. Curr Opin Biotechnol 2002; 13: 429–436.
Zaiss AK, Son S, Chang LJ . RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J Virol 2002; 76: 7209–7219.
Ramezani A, Hawley TS, Hawley RG . Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood 2003; 101: 4717–4724.
Aiuti A et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002; 296: 2410–2413.
Wu T et al. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol Ther 2000; 1: 285–293.
Horn PA et al. Lentivirus-mediated gene transfer into hematopoietic repopulating cells in baboons. Gene Therapy 2002; 9: 1464–1471.
Ragg S et al. Direct reversal of DNA damage by mutant methyltransferase protein protects mice against dose-intensified chemotherapy and leads to in vivo selection of hematopoietic stem cells. Cancer Res 2000; 60: 5187–5195.
Davis BM et al. Selection for G156A-O 6-methylguanine DNA methyltransferase gene-transduced hematopoietic progenitors and protection from lethality in mice treated with O6benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res 1997; 57: 5093–5099.
Davis BM et al. Limiting number of G156A O6-methylguanine DNA methyltransferase-transduced marrow progenitors repopulate nonmyeloablated mice after drug selection. Blood 2000; 95: 3078–3084.
Pegg AE et al. Mechanism of inactivation of human O6-alkylguanine-DNA alkyltransferase by O6-benzylguanine. Biochemistry 1993; 32: 11998–12006.
Davis BM et al. Characterisation of the P140K, PVP (138–140) MLK, and G156A O6-methylguanine-DNA methyltransferase mutants: implications for drug resistance gene therapy. Hum Gene Ther 1999; 10: 2769–2778.
Neff T et al. Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model. J Clin Invest 2003; 112: 1581–1588.
Zielske SP et al. In vivo selection of MGMT(P140K) lentivirus-transduced human NOD/SCID repopulating cells without pretransplant irradiation conditioning. J Clin Invest 2003; 112: 1561–1570.
Pollok KE et al. In vivo selection of human hematopoietic cells in a xenograft model using combined pharmacologic and genetic manipulations. Hum Gene Ther 2003; 14: 1703–1714.
Persons DA et al. Successful treatment of murine {beta}-thalassemia using in vivo selection of genetically modified, drug-resistant hematopoietic stem cells. Blood 2003; 102: 506–513.
Tutois et al. Erythropoietic protoporphyria in the house mouse. A recessive inherited ferrochelatase deficiency with anemia, photosensitivity, and liver disease. J Clin Invest 1991; 88: 1730–1736.
Sassa S . Hematological aspects of the porphyrias. Int J Hematol 2000; 1: 1–17.
Gouya L et al. The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet 2002; 30: 27–28.
Fontanellas A et al. Successful therapeutic effect in a mouse model of erythropoietic protoporphyria by partial genetic correction and fluorescence-based selection of hematopoietic cells. Gene Therapy 2001; 8: 618–626.
Pawliuk R et al. Long-term cure of the photosensitivity of murine erythropoietic protoporphyria by preselective gene therapy. Nat Med 1999; 7: 768–773.
Richard E et al. Gene therapy of a mouse model of protoporphyria with a self-inactivating erythroid-specific lentiviral vector without preselection. Mol Ther 2001; 4: 331–338.
Richard E et al. A bicistronic SIN-lentiviral vector containing G156A MGMT allows selection and metabolic correction of hematopoietic protoporphyric cell lines. J Gen Med 2003; 5: 737–747.
Halene S et al. Improved expression in hematopoietic and lymphoid cells in mice after transplantation of bone marrow transduced with a modified retroviral vector. Blood 1999; 94: 3349–3357.
Onodera M et al. Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood 1998; 91: 30–36.
Doerflinger N et al. Retroviral transfer and long-term expression of the adrenoleukodystrophy gene in human CD34+ cells. Hum Gene Ther 1998; 9: 1025–1036.
Dunbar CE, Kohn DB, Schiffmann R . Retroviral transfer of the glucocerebrosidase gene into CD34+ cells from patients with Gaucher disease: in vivo detection of transduced cells without myeloablation. Hum Gene Ther 1998; 9: 2629–2640.
Malech HL et al. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc Natl Acad Sci USA 1997; 94: 12133–12138.
Davis BM, Humeau L, Dropulic B . In vivo selection for human and murine hematopoietic cells transduced with a therapeutic MGMT lentiviral vector that inhibits HIV replication. Mol Ther 2004; 9: 160–172.
Puig T et al. Myeloablation enhances engraftment of transduced murine hematopoietic cells, but does not influence long-term expression of the transgene. Gene Therapy 2002; 9: 1472–1479.
Chen ZP et al. Enhanced antitumor activity of sarCNU in comparison to BCNU in an extraneuronal monoamine transporter positive human glioma xenograft model. J Neurooncol 1999; 44: 7–14.
Zielske SP, Gerson SL . SarCNU mediates selection of P140K methylguanine-DNA-methyltransferase transduced human CD34(+) cells in vitro. Blood Cells Mol Dis 2003; 31: 48–50.
Sawai N et al. Protection and in vivo selection of hematopoietic stem cells using temozolomide, O6-benzylguanine, and an alkyltransferase-expressing retroviral vector. Mol Ther 2001; 1: 78–87.
Moreau-Gaudry F et al. High-level erythroid-specific gene expression in primary human and murine hematopoietic cells with self-inactivating lentiviral vectors. Blood 2001; 98: 2664–2672.
Camadro JM, Labbe PA . A simple ferrochelatase assay. Biochimie 1981; 63: 463–468.
Acknowledgements
We thank Dr D Trono, (University of Geneva, Switzerland) for providing the SIN lentiviral backbone pRRL-PGK-WPRE, Dr D Kohn (Children's Hospital, Los Angeles, CA) for providing MND promoter, Dr P Malik for critical comments on the manuscript, Prof. H Fleury for providing RCR assays, Dr J Plassa and Dr A Chemin from Bergonié Institute Bordeaux for help in mice irradiation, and Isabelle Lamrissi-Garcia and Magalie Lalanne for technical assistance. This work has been supported by grants from Association Française contre les Myopathies (AFM) and Conseil Régional d’Aquitaine.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Richard, E., Robert, E., Cario-André, M. et al. Hematopoietic stem cell gene therapy of murine protoporphyria by methylguanine-DNA-methyltransferase-mediated in vivo drug selection. Gene Ther 11, 1638–1647 (2004). https://doi.org/10.1038/sj.gt.3302335
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302335
Keywords
This article is cited by
-
Correction of glycogenosis type 2 by muscle-specific lentiviral vector
In Vitro Cellular & Developmental Biology - Animal (2008)
-
Improved Human β-globin Expression from Self-inactivating Lentiviral Vectors Carrying the Chicken Hypersensitive Site-4 (cHS4) Insulator Element
Molecular Therapy (2007)