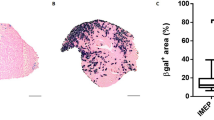
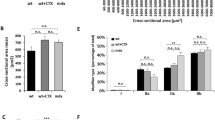
Abstract
The efficiency of plasmid gene transfer in skeletal muscle is significantly enhanced by pretreatment with hyaluronidase and the application of an electrical field to the muscle following the injection of plasmid DNA, a process referred to as electrotransfer. However, the presence of increased levels of connective tissue in muscular dystrophies, such as Duchenne muscular dystrophy (DMD), may affect the efficiency of this process. Here we demonstrate that the efficiency of electrotransfer is not affected by increased levels of connective tissue in the mdx mouse model of DMD and that any damage induced by the electrotransfer process is not exacerbated in the dystrophic phenotype. However, increasing the concentration of hyaluronidase does not improve transfection efficiencies further. Unlike direct injection of plasmid DNA, the efficiency of electrotransfer is not dependent upon the sex and age of mice used. The combined treatment of hyaluronidase and electrotransfer results in highly efficient gene transfer in dystrophic muscle with limited muscle damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Acsadi G et al. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature 1991; 352: 815–881.
Wolff JA et al. Direct gene transfer into mouse muscle in vivo. Science 1990; 247(Part 1): 1465–1468.
Davis HL, Whalen RG, Demeneix BA . Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression. Hum Gene Ther 1993; 4: 151–159.
Levy MY et al. Characterization of plasmid DNA transfer into mouse skeletal muscle: evaluation of uptake mechanism expression and secretion of gene products into blood. Gene Ther 1996; 3: 201–211.
Manthorpe M et al. Gene therapy by intramuscular injection of plasmid DNA: studies on firefly luciferase gene expression in mice. Hum Gene Ther 1993; 4: 419–431.
Wells DJ, Goldspink G . Age and sex influence expression of plasmid DNA directly injected into mouse skeletal muscle. FEBS Lett 1992; 306: 203–205.
Wells DJ et al. Evaluation of plasmid DNA for in vivo gene therapy: factors affecting the number of transfected fibers. J Pharm Sci 1998; 87: 763–768.
Mathiesen I . Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther 1999; 6: 508–514.
Mir LM et al. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad Sci III 1998; 321: 893–899.
Aihara H, Miyazaki J . Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 1998; 16: 867–870.
Vilquin J et al. Electrotransfer of naked DNA in the skeletal muscles of animal models of muscular dystrophies. Gene Ther 2001; 8: 1097–1107.
McMahon JM et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase – increased expression with reduced muscle damage. Gene Ther 2001; 8: 1264–1270.
Vicat JM et al. Muscle transfection by electroporation with high-voltage and short-pulse currents provides high-level and long-lasting gene expression. Hum Gene Ther 2000; 11: 909–916.
Mennuni C et al. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum Gene Ther 2002; 13: 355–365.
Danko I et al. High expression of naked plasmid DNA in muscles of young rodents. Hum Mol Genet 1997; 6: 1435–1443.
Jooss K, Turka LA, Wilson JM . Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther 1998; 5: 309–319.
Halbert CL et al. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998; 72: 9795–9805.
Mir LM et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA 1999; 96: 4262–4267.
Coulton GR et al. The mdx mouse skeletal muscle myopathy: I. A histological, morphometric and biochemical investigation. Neuropathol Appl Neurobiol 1988; 14: 53–70.
Budker V et al. Hypothesis: naked plasmid DNA is taken up by cells in vivo by a receptor-mediated process. J Gene Med 2000; 2: 76–88.
Satkauskas S et al. Slow accumulation of plasmid in muscle cells: supporting evidence for a mechanism of DNA uptake by receptor-mediated endocytosis. Mol Ther 2001; 4: 317–323.
Somiari S et al. Theory and in vivo application of electroporative gene delivery. Mol Ther 2000; 2: 178–187.
Vincent N et al. Long-term correction of mouse dystrophic degeneration by adenovirus-mediated transfer of a minidystrophin gene. Nat Genet 1993; 5: 130–134.
Deconinck N et al. Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc Natl Acad Sci USA 1996; 93: 3570–3574.
Clemens PR et al. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther 1996; 3: 965–972.
Jiao S et al. Direct gene transfer into nonhuman primate myofibers in vivo. Hum Gene Ther 1992; 3: 21–33.
Ferrer A . PhD thesis, University of London, 2001.
Ferrer A, Wells KE, Wells DJ . Immune responses to dystropin: implications for gene therapy of Duchenne muscular dystrophy. Gene Ther 2000; 7: 1439–1446.
Acknowledgements
We thank George Dickson (Royal Holloway, London) and Jim Owen (Royal Free Hospital, London) for the loan of the BTX electroporator and electrodes. This work was funded by the Medical Research Council of Great Britain, the Muscular Dystrophy Campaign and the Wellcome Trust.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gollins, H., McMahon, J., Wells, K. et al. High-efficiency plasmid gene transfer into dystrophic muscle. Gene Ther 10, 504–512 (2003). https://doi.org/10.1038/sj.gt.3301927
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301927
Keywords
This article is cited by
-
Physiological Characterization of Muscle Strength With Variable Levels of Dystrophin Restoration in mdx Mice Following Local Antisense Therapy
Molecular Therapy (2011)
-
Quantitative real-time PCR study on persistence of pDNA vaccine pVax-Hsp60 TM814 in beef muscles
Genetic Vaccines and Therapy (2008)
-
Codon and mRNA Sequence Optimization of Microdystrophin Transgenes Improves Expression and Physiological Outcome in Dystrophic mdx Mice Following AAV2/8 Gene Transfer
Molecular Therapy (2008)
-
Enhanced effect of microdystrophin gene transfection by HSV-VP22 mediated intercellular protein transport
BMC Neuroscience (2007)