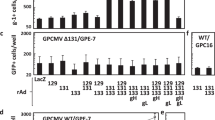
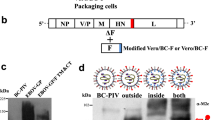
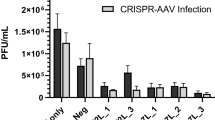
Abstract
Direct protein delivery is an emerging technology in vaccine development and gene therapy. We could previously show that subviral dense bodies (DB) of human cytomegalovirus (HCMV), a beta-herpesvirus, transport viral proteins into target cells by membrane fusion. Thus these non-infectious particles provide a candidate delivery system for the prophylactic and therapeutic application of proteins. Here we provide proof of principle that DB can be modified genetically. A 55 kDa fusion protein consisting of the green fluorescent protein and the neomycin phosphotransferase could be packed in and delivered into cells by recombinant DB in a functional fashion. Furthermore, transfer of protein into fibroblasts and dendritic cells by DB was efficient, leading to exogenous loading of the MHC-class I antigen presentation pathway. Thus, DB may be a promising basis for the development of novel vaccine strategies and therapeutics based on recombinant polypeptides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sigal LJ et al. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature 1999; 398: 77–80.
Arrode G et al. Incoming human cytomegalovirus pp65 (UL83) contained in apoptotic infected fibroblasts is cross-presented to CD8(+) T cells by dendritic cells. J Virol 2000; 74: 10 018–10 024.
Irmiere A, Gibson W . Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 1983; 130: 118–133.
Schmolke S et al. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol. 1995; 69: 1071–1078.
Pepperl S et al. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J Virol. 2000; 74: 6132–6146.
Topilko A, Michelson S . Hyperimmediate entry of human cytomegalovirus virions and dense bodies into human fibroblasts. Res Virol 1994; 145: 75–82.
Wolff D, Jahn G, Plachter B . Generation and effective enrichment of selectable human cytomegalovirus mutants using site-directed insertion of the neo gene. Gene 1993; 130: 167–173.
Riegler S et al. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J Gen Virol 2000; 81: 393–399.
Pepperl-Klindworth S, Frankenberg N, Plachter B . Development of novel vaccine strategies against human cytomegalovirus infection based on subviral particles. J Clin Virol, in press.
Sherman LA et al. Selecting T cell receptors with high affinity for self-MHC by decreasing the contribution of CD8. Science 1992; 258: 815–818.
Vitiello A et al. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human–mouse class I major histocompatibility complex. J Exp Med 1991; 173: 1007–1015.
Wills MR et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65- specific CTL. J Virol 1996; 70: 7569–7579.
Diamond DJ et al. Development of a candidate HLA A*0201 restricted peptide-based vaccine against human cytomegalovirus infection. Blood 1997; 90: 1751–1767.
Theodore L et al. Intraneuronal delivery of protein kinase C pseudosubstrate leads to growth cone collapse. J Neurosci 1995; 15: 7158–7167.
Derossi D et al. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem 1996; 271: 18 188–18 193.
Phelan A, Elliott G, O'Hare P . Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol 1998; 16: 440–443.
Green M, Loewenstein PM . Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988; 55: 1179–1188.
Frankel AD, Pabo CO . Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988; 55: 1189–1193.
Morris MC et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 2001; 19: 1173–1176.
Schwarze SR et al. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 1999; 285: 1569–1572.
Walev I et al. Delivery of proteins into living cells by reversible membrane permeabilization with streptolysin-O. Proc Natl Acad Sci USA 2001; 98: 3185–3190.
Ford KG et al. Protein transduction: an alternative to genetic intervention? Gene Ther. 2001; 8: 1–4.
Morris MC et al. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 2001; 19: 1173–1176.
Elek SD, Stern H . Development of a vaccine against mental retardation caused by cytomegalovirus infection in utero. Lancet 1974; 1: 1–5.
Neff BJ et al. Clinical and laboratory studies of live cytomegalovirus vaccine Ad-169. Proc Soc Exp Biol Med 1979; 160: 32–37.
Starr SE et al. Specific cellular and humoral immunity after immunization with live Towne strain cytomegalovirus vaccine. J Infect Dis 1981; 143: 585–589.
Bachmair A, Finley D, Varshavsky A . In vivo half-life of a protein is a function of its amino-terminal residue. Science 1986; 234: 179–186.
Pickart CM, Rose IA . Ubiquitin carboxyl-terminal hydrolase acts on ubiquitin carboxyl-terminal amides. J Biol Chem 1985; 260: 7903–7910.
Chee MS et al. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Topics Microbiol Immunol 1990; 154: 125–169.
Schmolke S et al. The dominant phosphoprotein pp65 (UL83) of human cytomegalovirus is dispensable for growth in cell culture. J Virol 1995; 69: 5959–5968.
Sallusto F, Lanzavecchia A . Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179: 1109–1118.
Holtappels R et al. Enrichment of immediate-early 1 (m123/pp89) peptide-specific CD8 T cells in a pulmonary CD62L(lo) memory-effector cell pool during latent murine cytomegalovirus infection of the lungs. J Virol 2000; 74: 11 495–11 503.
Jonuleit H et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27: 3135–3142.
Herr W et al. Identification of naturally processed and HLA-presented Epstein-Barr virus peptides recognized by CD4(+) or CD8(+) T lymphocytes from human blood. Proc Natl Acad Sci USA 1999; 96: 12 033–12 038.
Acknowledgements
The collaboration with Matthias Theobald, Mainz, and his colleagues in generating CTL-clones in transgenic mice is gratefully appreciated. The donations of monoclonal antibodies by William Britt, Birmingham, AL and of cosmid clones of HCMV by Christian Sinzger, Tübingen are acknowledged. We thank Ulrike Stapf for technical assistance. This work was supported by Sonderforschungsbereich 490, individual grant B2 and Sonderforschungsbereich 510, individual grant B3 (S. Riegler).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pepperl-Klindworth, S., Frankenberg, N., Riegler, S. et al. Protein delivery by subviral particles of human cytomegalovirus. Gene Ther 10, 278–284 (2003). https://doi.org/10.1038/sj.gt.3301879
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301879
Keywords
This article is cited by
-
Nucleocytoplasmic shuttling and CRM1-dependent MHC class I peptide presentation of human cytomegalovirus pp65
Medical Microbiology and Immunology (2012)
-
Human cytomegalovirus protein pp65: an efficient protein carrier system into human dendritic cells
Gene Therapy (2008)
-
Refinement of strategies for the development of a human cytomegalovirus dense body vaccine
Medical Microbiology and Immunology (2008)