Abstract
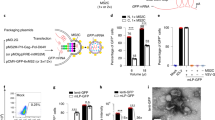
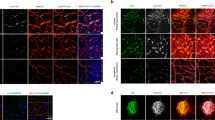
Ischemic retinal diseases, such as diabetic retinopathy, retinopathy of prematurity, and age-related macular degeneration, are a major cause of blindness worldwide. Angiostatin is an internal peptide fragment of plasminogen that inhibits endothelial proliferation in vitro and tumor growth in vivo. We now demonstrate that HIV vector encoding angiostatin (HIV-angiostatin) can inhibit retinal neovascularization in a mouse model of proliferative retinopathy. Intravitreal injections of HIV-angiostatin led to stable expression of the angiostatin gene in retinal tissue. Retinal neovascularization was histologically quantitated by a masked protocol. Retinal neovascularization in the eye injected with HIV-angiostatin was reduced in 90% (9/10; P=0.025) of animals, compared with the eye injected with phosphate-buffered saline. Reduction of histologically evident neovascular nuclei per 6-μm section averaged 68%, with maximal inhibitory effects of 87%. Neovascularization was not reduced in the eyes injected with HIV vector encoding enhanced green fluorescent protein. This is the first report that HIV-angiostatin can reduce neovascular cell nuclei in a murine proliferative retinopathy model. These data suggest that the anti-angiogenic activity of angiostatin has therapeutic potential for the treatment of retinal neovascularization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Klein R, klein B . Vision disorders in diabetes. In: National Diabetes Data Group (ed). Diabetes in America. 2nd edn., National Institutes of Health: Washington, DC, 1995; pp. 305–321.
The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol 1976; 81(4): 383–396.
Early Treatment Diabetic Retinopathy Study Group. Ophthalmology 1991; 98: 741–840.
O'Reilly MS et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–328.
Tanaka T, Cao Y, Folkman J, Fine HA . Viral vector-targeted antiangiogenic gene therapy utilizing an angiostatin complementary DNA. Cancer Res 1998; 58: 3362–3369.
Gyorffy S, Palmer K, Gauldie J . Adenoviral vector expressing murine angiostatin inhibits a model of breast cancer metastatic growth in the lungs of mice. Am J Pathol 2001; 159: 1137–1147.
Indraccolo S et al. Effects of angiostatin gene transfer on functional properties and in vivo growth of Kaposi's sarcoma cells. Cancer Res 2001; 61: 5441–5446.
Mori K et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci 2002; 43: 1994–2000.
Lai CC et al. Suppression of choroidal neovascularization by adeno-associated virus vector expressing angiostatin. Invest Ophthalmol Vis Sci 2001; 42: 2401–2407.
Bainbridge JW et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther 2002; 9: 320–326.
Lai YK et al. Potential long-term inhibition of ocular neovascularisation by recombinant adeno-associated virus-mediated secretion gene therapy. Gene Ther 2002; 9: 804–813.
Mori K et al. Inhibition of choroidal neovascularization by intravenous injection of adenoviral vectors expressing secretable endostatin. Am J Pathol 2001; 159: 313–320.
Smith LE et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994; 35: 101–111.
Gao G et al. Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5. J Biol Chem 2002; 277: 9492–9497.
McDonald HR, Schatz H . Macular edema following panretinal photocoagulation. Retina 1985; 5: 5–10.
Lucas R et al. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood 1998; 92: 4730–4741.
Troyanovsky B et al. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J Cell Biol 2001; 152: 1247–1254.
Tanaka K et al. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin Cancer Res 2002; 8: 1125–1131.
Aiello LP et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487.
Lopez PF et al. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1996; 37: 855–868.
Pierce EA et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 1995; 92: 905–909.
Dawson DW et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999; 285: 245–248.
Tombran-Tink J, Johnson LV . Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci 1989; 30: 1700–1707.
Gao G et al. Unbalanced expression of VEGF and PEGF in ischemia-induced retinal neovascularization. FEBS Lett 2001; 489: 270–276.
Spranger J et al. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia 2000; 43: 1404–1407.
O'Reilly MS et al. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996; 2: 689–692.
Berger AS et al. Intravitreal sustained release corticosteroid-5-fluoruracil conjugate in the treatment of experimental proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci 1996; 37: 2318–2325.
Yang CS et al. An intravitreal sustained-release triamcinolone and 5-fluorouracil codrug in the treatment of experimental proliferative vitreoretinopathy. Arch Ophthalmol 1998; 116: 69–77.
Li T et al. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci 1994; 35: 2543–2549.
Murata T et al. Retrovirus-mediated gene transfer targeted to retinal photocoagulation sites. Diabetologia 1998; 41: 500–506.
Ferrari FK et al. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol 1996; 70: 3227–3234.
Igarashi T et al. New strategy for in vivo transgene expression in corneal epithelial progenitor cells, submitted for publication. Current Eye Research 2002; 24: 46–50.
Shimada T et al. Targeted and highly efficient gene transfer into CD4+ cells by a recombinant human immunodeficiency virus retroviral vector. J Clin Invest 1991; 88: 1043–1047.
Emi N, Friedmann T, Yee JK . Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol 1991; 65: 1202–1207.
Uchida N et al. HIV, but not murine leukemia virus, vectors mediate high efficiency gene transfer into freshly isolated G0/G1 human hematopoietic stem cells. Proc Natl Acad Sci USA 1998; 95: 11 939–11 944.
Zufferey R et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat Biotechnol 1997; 15: 871–875.
Miyoshi H et al. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA 1997; 94: 10 319–10 323.
Takahashi M et al. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol 1999; 73: 7812–7816.
Cao Y . Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol 2001; 33: 357–369.
Orimo H et al. Association between single nucleotide polymorphisms in the hMSH3 gene and sporadic colon cancer with microsatellite instability. J Hum Genet 2000; 45: 228–230.
Miyoshi H et al. Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Niwa H, Yamamura K, Miyazaki J . Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991; 108: 193–199.
Naldini L et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Miyake K et al. Selective killing of human immunodeficiency virus-infected cells by targeted gene transfer and inducible gene expression using a recombinant human immunodeficiency virus vector. Hum Gene Ther 2001; 12: 227–233.
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning: A Laboratory Manual. Cold spring Harbor Laboratory Press: Cold Spring Harbor NY, 1989, pp. 1860–1875.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Igarashi, T., Miyake, K., Kato, K. et al. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther 10, 219–226 (2003). https://doi.org/10.1038/sj.gt.3301878
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301878
Keywords
This article is cited by
-
Updates on Gene Therapy for Diabetic Retinopathy
Current Diabetes Reports (2020)
-
Novel stem cell and gene therapy in diabetic retinopathy, age related macular degeneration, and retinitis pigmentosa
International Journal of Retina and Vitreous (2019)
-
Lung fibroblasts express a miR-19a-19b-20a sub-cluster to suppress TGF-β-associated fibroblast activation in murine pulmonary fibrosis
Scientific Reports (2018)
-
A Comprehensive Review of Retinal Gene Therapy
Molecular Therapy (2013)
-
Ocular gene delivery using lentiviral vectors
Gene Therapy (2012)