Abstract
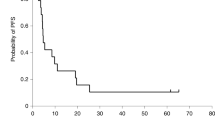
A silicone formulation of ganciclovir (GCV-pellet) was developed to enhance the cytotoxic effects of herpes simplex virus thymidine kinase suicide gene therapy. The effectiveness of this drug delivery system was assessed in a rat 9L gliosarcoma model. The GCV-pellets (1 mm in length and in diameter) used in this experiment contained a total amount of 0.15 mg of GCV. In vitro experiments demonstrated that GCV was gradually released over a period of 7 days. Five days after stereotactic tumor inoculation into the right caudate nucleus, a herpes simplex virus type 1 (HSV-1) vector expressing herpes simplex virus thymidine kinase (HSV-tk) (T1, 2×106 pfu) was administered at the same location. The survival rate of the group treated with the GCV-pellet was compared with that of the T1 group injected intraperitoneally (IP) with GCV (30 mg/kg/day for 7 days). The GCV-pellet-treated group had a significantly prolonged survival (a median of more than 80 days) compared with the GCV IP group (a median of 65 days) and with control groups (P<0.05). The control groups (untreated or receiving only the virus vector) had a survival of 35–38 days. The survival rate of the GCV-pellet group over 80 days was 75%, and all the rats that survived more than 80 days and did not show tumors upon histological examination of the brain were deemed cured. No toxic effects or immunological reactions were observed histologically around the pellet in brain sections from the rats treated with the GCV-pellet. After GCV-pellet inoculation into the tumor, drug concentrations were kept at 1–10 μg/g tissue for 3–4 days. When the same dose of GCV (0.15 mg) in aqueous solution was injected into the tumor, GCV concentrations reached a peak of 0.5 mg/g tissue after 30 min and decreased below measurable level within 12 h. After IP injections of 3 mg GCV, GCV concentrations in the tumor reached a peak of 5.7 μg/g tissue after 30 min and also decreased below measurable level within 12 h. This sustained release of a low and effective GCV dose with the silicone formulation significantly prolonged survival in combinations with HSV-tk expression if compared to IP administration of GCV. Histological examination suggests that the treatment appears to be safe.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Arcicasa M et al. Results of three consecutive combined treatments for malignant gliomas. Ten-year experience at a single institution Am J Clin Oncol 1994 17: 437–443
Mahaley Jr MS et al. National survey of patterns of care for brain-tumor patients J Neurosurg 1989 71: 826–836
Ushio Y . Treatment of gliomas in adults Curr Opin Oncol 1991 3: 467–475
Chang SM, Prados MD . Chemotherapy for gliomas Curr Opin Oncol 1995 7: 207–213
Kaye AH, Laidlaw JD . Chemotherapy for gliomas Curr Opin Neurol Neurosurg 1992 5: 526–533
Barba D, Hardin J, Sadelain M, Gage FH . Development of antitumor immunity following thymidine kinase-mediated killing of experimental brain tumors Proc Natl Acad Sci USA 1994 91: 4348–4352
Chen SH et al. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo Proc Natl Acad Sci USA 1994 91: 3054–3057
Perez-Cruet MJ et al. Adenovirus-mediated gene therapy of experimental gliomas J Neurosci Res 1994 39: 506–511
Ram Z et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells Nat Med 1997 12: 1354–1361
Rainov NG . A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme Hum Gene Ther 2000 11: 2389–2401
Marconi P et al. Connexin 43-enhanced suicide gene therapy using herpesviral vectors Mol Ther 2000 1: 71–81
Moriuchi S et al. Enhanced tumor cell killing in the presence of ganciclovir by herpes simplex virus type 1 vector-directed coexpression of human tumor necrosis factor-alpha and herpes simplex virus thymidine kinase Cancer Res 1998 58: 5731–5737
Cirenei N et al. In vitro and in vivo effects of retrovirus-mediated transfer of the connexin 43 gene in malignant gliomas: consequences for HSVtk/GCV anticancer gene therapy Gene Ther 1998 5: 1221–1226
Hasegawa Y et al. Avoidance of bone marrow suppression using A-5021 as a nucleoside analog for retrovirus-mediated herpes simplex virus type I thymidine kinase gene therapy Cancer Gene Ther 2000 4: 557–562
Kajihara M et al. Development of new drug delivery system for protein drugs using silicone (I) J Control Rel 2000 66: 49–61
Kajihara M et al. Development of new drug delivery system for protein drugs using silicone (II) J Control Rel 2001 73: 279–291
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays J Immunol Methods 1983 65: 55–63
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miura, F., Moriuchi, S., Maeda, M. et al. Sustained release of low-dose ganciclovir from a silicone formulation prolonged the survival of rats with gliosarcomas under herpes simplex virus thymidine kinase suicide gene therapy. Gene Ther 9, 1653–1658 (2002). https://doi.org/10.1038/sj.gt.3301860
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301860
Keywords
This article is cited by
-
Genetic strategies for brain tumor therapy
Cancer Gene Therapy (2006)