Abstract
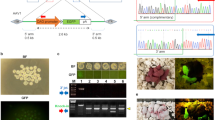
Our aim is to develop a simple gene transfer method into egg cylinder and mid-gestational murine embryos. We examined whether plasmid/lipid complexes injected into the tail veins of pregnant transgenic mice can be transferred to fetuses at E 4.5–13.5. When pregnant CETZ-17 mice carrying a transgene consisting of a ubiquitous promoter, floxed EGFP/CAT and the LacZ gene, were injected with a Cre expression vector DNA/lipid complex, Cre-mediated excision of the transgenes, as evaluated by X-gal staining, occurred in 10–50% of fetuses treated at E 11.5–13.5. Although younger embryos remained unstained, PCR analysis revealed low levels of the Cre vector DNA and recombined transgene. To examine the fate of a solution given intravenously, we injected trypan blue or fluorescence-labeled plasmid DNA/lipid complexes into females at E 5.5–11.5 and E 6.5, respectively. Both collected in the visceral endoderm (VE) lineage, but were undetectable in the embryo proper. These findings suggest that substances in maternal blood are delivered to post-implantation embryos via cells of the VE lineage and placenta, but that most are trapped in the VE. If significantly improved, gene transfer to fetuses by injection into the maternal circulation may become a promising tool in fetal gene therapy and embryological studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Douar A-M et al. Foetal gene delivery in mice by intra-amniotic administration of retroviral producer cells and adenovirus Gene Therapy 1997 4: 883–890
Mitchell M et al. Long-term gene transfer to mouse fetuses with recombinant adenovirus and adeno-associated virus (AAV) vectors Gene Therapy 2000 23: 1986–1992
Zhu N et al. Systemic gene expression after intravenous DNA delivery in adult mice Science 1993 261: 209–211
Liu F, Qi H, Huang L, Liu D . Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration Gene Therapy 1997 4: 517–523
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery Nat Biotechnol 1997 15: 167–173
Templeton NS et al. Improved DNA:liposome complexes for increased systemic delivery and gene expression Nat Biotechnol 1997 15: 647–652
Larson JE et al. Reversal of cystic fibrosis phenotype in mice by gene therapy in utero Lancet 1997 349: 619–620
Papaioannou VE . In utero manipulation Copp AJ, Cockroft DL (eds); Postimplantation Mammalian Embryos IRL Press at Oxford University Press 1990 pp 61–80
Woo YJ et al. In utero cardiac gene transfer via intraplacental delivery of recombinant adenovirus Circulation 1997 96: 3561–3569
Türkay A, Saunders TL, Kurachi K . Intrauterine gene transfer: gestational stage-specific gene delivery in mice Gene Therapy 1999 6: 1685–1694
McCray PB Jr . Adenoviral-mediated gene transfer to fetal pulmonary epithelia in vitro and in vivo J Clin Invest 1995 95: 2620–2632
Schachtner S et al. Temporally regulated expression patterns following in utero adenovirus-mediated gene transfer Gene Therapy 1999 6: 1249–1257
Themis M et al. Successful expression of beta-galactosidase and factor IX transgenes in fetal and neonatal sheep after ultrasound-guided percutaneous adenovirus vector administration into the umbilical vein Gene Therapy 1999 6: 1239–1248
Tsukamoto M et al. Gene transfer and expression in progeny after intravenous DNA injection into pregnant mice Nat Genet 1995 9: 243–248
Ochiya T et al. Evaluation of cationic liposome suitable for gene transfer into pregnant animals Biochem Biophys Res Commun 1999 258: 358–365
Okuda K et al. Transplacental genetic immunization after intravenous delivery of plasmid DNA to pregnant mice J Immunol 2001 167: 5478–5484
Nagy A . Cre recombinase: the universal reagent for genome tailoring Genesis 2000 26: 99–109
Sato M et al. New approach to cell lineage analysis in mammals using the Cre-loxP system Mol Reprod Dev 2000 56: 34–44
Niwa H, Yamamura K, Miyazaki J . Efficient selection for high-expression transfectants with a novel eukaryotic vector Gene 1991 108: 193–200
Wiesenhofer B, Kaufmann WA, Humpel C . Improved lipid-mediated gene transfer in C6 glioma cells and primary glial cells using FuGene J Neurosci Meth 1999 92: 145–152
Jeker M et al. Mouse thyroid primary culture Biochem Biophys Res Commun 1999 257: 511–515
Araki K, Araki M, Miyazaki J, Vassalli P . Site-specific recombination of a transgene in fertilized eggs by transient expression of Cre recombinase Proc Natl Acad Sci USA 1995 92: 160–164
Ishii M et al. Embryonic expression of MHC class I heavy and light chains in transgenic mice Endocr J 1994 41: S9–S16
Sato M et al. Cytomegalovirus enhancer/chicken β-actin promoter confers constitutive and ubiquitous transgene expression in vivo Transgenics 1997 2: 153–159
Kawarabayashi T et al. Accumulation of β-amyloid fibrils in pancreas of transgenic mice Neurobiol Aging 1996 17: 215–222
Sato M et al. Usefulness of double gene construct for rapid identification of transgenic mice exhibiting tissue-specific gene expression Mol Reprod Dev 2001 60: 446–456
Williams KE et al. Inhibition of pinocytosis in rat yolk sac by trypan blue Teratology 1976 14: 343–354
Roberts G, Williams KE, Lloyd JB . Mechanism of stimulation of pinocytosis by trypan blue Chem Biol Interact 1980 32: 305–310
Beckman DA, Lloyd JB, Brent RL . Investigations into mechanisms of amino acid supply to the rat embryo using whole-embryo culture Int J Dev Biol 1997 41: 315–318
Terasawa Y et al. Apolipoprotein B-related gene expression and ultrastructural characteristics of lipoprotein secretion in mouse yolk sac during embryonic development J Lipid Res 1999 40: 1967–1977
Shalaby F et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice Nature 1995 376: 62–66
Ishikawa T et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis Development 2001 128: 25–33
Belaoussoff M, Farrington SM, Baron MH . Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo Development 1998 125: 5009–5018
Shawlot W et al. Lim1 is required in both primitive streak-derived tissues and visceral endoderm for head formation in the mouse Development 1999 126: 4925–4932
Gaensler KM et al. Fetal gene transfer by transuterine injection of cationic liposome-DNA complexes Nat Biotechnol 1999 17: 1188–1192
Templeton NS et al. New directions in liposome gene delivery Mol Biotechnol 1999 11: 175–180
Yamazaki Y et al. In vivo gene transfer to mouse spermatogenic cells by deoxyribonucleic acid injection into seminiferous tubules and subsequent electroporation Biol Reprod 1998 59: 1439–1444
Blin N, Stafford DW . A general method for isolation of high molecular weight DNA from eukaryotes Nucleic Acids Res 1976 3: 2303–2308
Sato M, Iwase R, Kasai K, Tada N . Direct injection of foreign DNA into mouse testis as a possible alternative of sperm-mediated gene transfer Animal Biotech 1994 5: 19–31
Sternberg N, Hamilton D . Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites J Mol Biol 1981 150: 467–486
Kalnins A, Otto K, Rüther U, Muller-Hill B . Sequence of the LacZ gene of Escherichia coli EMBO J 1983 2: 593–597
van Ooyen A, van der Berg J, Mantei N, Weissmann C . Comparison of total sequence of a cloned rabbit β-globin gene and its flanking regions with a homologous mouse sequence Science 1979 206: 337–344
Bernstine EG, Hooper ML, Grandchamp S, Ephrussi B . Alkaline phosphate activity in mouse teratoma Proc Natl Acad Sci USA 1973 70: 3899–3902
Sato M, Watanabe T, Kimura M . Embryo transfer via oviductal wall: an alternative method for efficient production of transgenic mice Transgenics 1999 2: 383–389
Sato M, Tada N . Preferential expression of osteocalcin-related protein mRNA in gonadal tissues of male mice Biochem Biophys Res Commun 1995 215: 412–421
Tokunaga K et al. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA Nucleic Acids Res 1986 14: 2829
Kost TA, Theodorakis N, Hughes SH . The nucleotide sequence of the chick cytoplasmic β-actin gene Nucleic Acids Res 1983 11: 8287–8301
Acknowledgements
We thank Drs Kimi Araki (Kumamoto University) and Jun-ichi Miyazaki (Osaka University) for providing us with CAG-Cre transgenic mice and pCAG-CAT-lacZ plasmid, respectively, and Dr Osamu Tanaka (Tokai University) for discussion. MS and MK were supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kikuchi, N., Nakamura, S., Ohtsuka, M. et al. Possible mechanism of gene transfer into early to mid-gestational mouse fetuses by tail vein injection. Gene Ther 9, 1529–1541 (2002). https://doi.org/10.1038/sj.gt.3301818
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301818
Keywords
This article is cited by
-
In vivo genome editing targeted towards the female reproductive system
Archives of Pharmacal Research (2018)
-
Nucleic acids delivery methods for genome editing in zygotes and embryos: the old, the new, and the old-new
Biology Direct (2016)
-
Kidney-specific expression of GFP by in-utero delivery of pseudotyped adeno-associated virus 9
Molecular Therapy - Methods & Clinical Development (2014)
-
A new mouse model for renal lesions produced by intravenous injection of diphtheria toxin A-chain expression plasmid
BMC Nephrology (2004)