Abstract
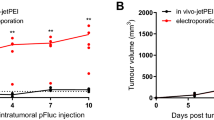
Interstitial transport is a crucial step in plasmid DNA-based gene therapy. However, interstitial diffusion of large nucleic acids is prohibitively slow. Therefore, we proposed to facilitate interstitial transport of DNA via pulsed electric fields. To test the feasibility of this approach to gene delivery, we developed an ex vivo technique to quantify the magnitude of DNA movement due to pulsed electric fields in two tumor tissues: B16.F10 (a mouse melanoma) and 4T1 (a mouse mammary carcinoma). When the pulse duration and strength were 50 ms and 233 V/cm, respectively, we found that the average plasmid DNA movements per 10 pulses were 1.47 μm and 0.35 μm in B16.F10 and 4T1 tumors, respectively. The average plasmid DNA movements could be approximately tripled, ie to reach 3.69 μm and 1.01 μm, respectively, when the pulse strength was increased to 465 V/cm. The plasmid DNA mobility was correlated with the tumor collagen content, which was approximately eight times greater in 4T1 than in B16.F10 tumors. These data suggest that electric field can be a powerful driving force for improving interstitial transport of DNA during gene delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jain RK . Transport of molecules in the tumor interstitium: a review Cancer Res 1987 47: 3039–3051
Titomirov AV, Sukharev S, Kistanova E . In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA Biochim Biophys Acta 1991 1088: 131–134
Belehradek J Jr et al. Electropermeabilization of cells in tissues assessed by the qualitative and quantitative electroloading of bleomycin Biochim Biophys Acta 1994 1190: 155–163
Jaroszeski MJ, Gilbert RA, Heller R . In vivo antitumor effects of electrochemotherapy in a hepatoma model Biochim Biophys Acta 1997 1334: 15–18
Suzuki T et al. Direct gene transfer into rat liver cells by in vivo electroporation FEBS Lett 1998 425: 436–440
Belehradek M et al. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial Cancer 1993 72: 3694–3700
Hofmann GA et al. Electroporation therapy: a new approach for the treatment of head and neck cancer IEEE Trans Biomed Eng 1999 46: 752–759
Canatella PJ et al. Quantitative study of electroporation-mediated molecular uptake and cell viability Biophys J 2001 80: 755–764
Widera G et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo J Immunol 2000 164: 4635–4640
Li S et al. Intramuscular electroporation delivery of IFN-alpha gene therapy for inhibition of tumor growth located at a distant site Gene Therapy 2001 8: 400–407
Lohr F et al. Effective tumor therapy with plasmid-encoded cytokines combined with in vivo electroporation Cancer Res 2001 61: 3281–3284
Edwards CA, O'Brien WD Jr . Modified assay for determination of hydroxyproline in a tissue hydrolyzate Clin Chim Acta 1980 104: 161–167
Netti PA et al. Role of extracellular matrix assembly in interstitial transport in solid tumors Cancer Res 2000 60: 2497–2503
Pluen A et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs subcutaneous tumors Proc Natl Acad Sci USA 2001 98: 4628–4633
Pluen A et al. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations Biophys J 1999 77: 542–552
Sikes ML et al. In vivo gene transfer into rabbit thyroid follicular cells by direct DNA injection Hum Gene Ther 1994 5: 837–844
Lew D et al. Cancer gene therapy using plasmid DNA: pharmacokinetic study of DNA following injection in mice Hum Gene Ther 1995 6: 553–564
Meyer KB et al. Intratracheal gene delivery to the mouse airway: characterization of plasmid DNA expression and pharmacokinetics Gene Therapy 1995 2: 450–460
Yovandich J et al. Gene transfer to synovial cells by intra-articular administration of plasmid DNA Hum Gene Ther 1995 6: 603–610
Dimitrov DS, Sowers AE . Membrane electroporation – fast molecular exchange by electroosmosis Biochim Biophys Acta 1990 1022: 381–392
Klenchin VA et al. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis Biophys J 1991 60: 804–811
Sukharev SI et al. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores Biophys J 1992 63: 1320–1327
Sabelnikov AG . Nucleic acid transfer through cell membranes: towards the underlying mechanisms Prog Biophys Mol Biol 1994 62: 119–152
Mir LM et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses Proc Natl Acad Sci USA 1999 96: 4262–4267
Bettan M et al. High-level protein secretion into blood circulation after electric pulse-mediated gene transfer into skeletal muscle Mol Ther 2000 2: 204–210
Neumann E et al. Gene transfer into mouse lyoma cells by electroporation in high electric fields EMBO J 1982 1: 841–845
Knutson JC, Yee D . Electroporation: parameters affecting transfer of DNA into mammalian cells Anal Biochem 1987 164: 44–52
Winterbourne DJ et al. Electric shock-mediated transfection of cells. Characterization and optimization of electrical parameters Biochem J 1988 251: 427–434
Lucas ML, Heller R . Immunomodulation by electrically enhanced delivery of plasmid DNA encoding IL-12 to murine skeletal muscle Mol Ther 2001 3: 47–53
Lucas ML et al. In vivo electroporation using an exponentially enhanced pulse: a new waveform DNA Cell Biol 2001 20: 183–188
Akerman B . Effects of supercoiling in electrophoretic trapping of circular DNA in polyacrylamide gels Biophysical J 1998 74: 3140–3151
Serwer P . Sieving of double-stranded DNA during agarose gel electrophoresis Electrophoresis 1989 10: 327–331
Acknowledgements
DAZ is supported by a NIH biotechnology training grant for the Center for Cellular and Biosurface Engineering at Duke University.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaharoff, D., Barr, R., Li, CY. et al. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther 9, 1286–1290 (2002). https://doi.org/10.1038/sj.gt.3301799
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301799
Keywords
This article is cited by
-
The impact of impaired DNA mobility on gene electrotransfer efficiency: analysis in 3D model
BioMedical Engineering OnLine (2021)
-
New Insights into the Mechanisms of Gene Electrotransfer – Experimental and Theoretical Analysis
Scientific Reports (2015)
-
Efficient In Vitro Electropermeabilization of Reconstructed Human Dermal Tissue
The Journal of Membrane Biology (2015)
-
In Vivo Molecular Imaging and Histological Analysis of Changes Induced by Electric Pulses Used for Plasmid DNA Electrotransfer to the Skin: A Study in a Dorsal Window Chamber in Mice
The Journal of Membrane Biology (2012)
-
Electroporation increases antitumoral efficacy of the bcl-2 antisense G3139 and chemotherapy in a human melanoma xenograft
Journal of Translational Medicine (2011)