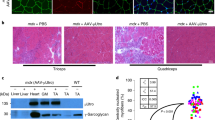
Abstract
The delivery of antigenic proteins in the context of a DNA vaccine leads to the intracellular synthesis of antigen and the induction of both humoral and cellular immune responses. Subsequent to immune activation, any transfected cell expressing the immunogenic protein should, by the rules of immunology, become a legitimate target for removal by immune-mediated mechanisms. Herein, we have used an indirect assay of myocyte integrity following intra-muscular (i.m.) delivery of a DNA vaccine, in mice with various immune deficiencies, to determine which immunological mechanisms may be involved in destruction of antigen-expressing cells. We demonstrate that destruction of antigen-expressing myocytes following i.m. injection of a DNA vaccine is dependent on major histocompatability complex (MHC) class II restricted CD4+ T cell activation, but is not mediated solely by MHC I-restricted or perforin-mediated lysis and appears to have a component that is antibody-mediated. Although we studied myocytes, the results likely represent what happens to any transfected cell expressing a foreign antigen. This study underscores the ability of DNA vaccines at inducing antigen-specific immune responses that include a number of effector mechanisms. From the perspective of gene therapy, this study highlights the significance of immune activation when considering strategies where maintenance of therapeutic gene expression is desired. Gene Therapy (2001) 8, 1395–1400.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davis HL, Michel ML, Whalen RG . DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody Hum Molec Genet 1993 2: 1847–1851
Davis HL et al. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen Vaccine 1994 12: 1503–1509
Davis HL, Schirmbeck R, Reimann J, Whalen RG . DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein Hum Gene Ther 1995 6: 1447–1456
Kowalczyk DW, Ertl HC . Immune responses to DNA vaccines Cell Mol Life Sci 1999 55: 751–770
Davis HL, Mancini M, Michel ML, Whalen RG . DNA-mediated immunization to hepatitis B surface antigen: longevity of primary response and effect of boost Vaccine 1996 14: 910–915
Wolff JA et al. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle Hum Mol Genet 1992 1: 363–369
Cheng L, Ziegelhoffer PR, Yang NS . In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment Proc Natl Acad Sci USA 1993 90: 4455–4459
Davis HL, Millan CL, Watkins SC . Immune-mediated destruction of transfected muscle fibers after direct gene transfer with antigen-expressing plasmid DNA Gene Therapy 1997 4: 181–188
Zijlstra M et al. Beta 2-microglobulin deficient mice lack CD4–8+ cytolytic T cells Nature 1990 344: 742–746
Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH . Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice Science 1991 253: 1417–1420
Klavinskis LS, Barnfield C, Gao L, Parker S . Intranasal immunization with plasmid DNA–lipid complexes elicits mucosal immunity in the female genital and rectal tracts J Immunol 1999 162: 254–262
Schirmbeck R et al. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice J Virol 1995 69: 5929–5934
Harty JT, Tvinnereim AR, White DW . CD8+ T cell effector mechanisms in resistance to infection Annu Rev Immunol 2000 18: 275–308
Gholson CF, Siddiqui A, Vierling JM . Cell surface expression of hepatitis B surface and core antigens in transfected rat fibroblast cell lines Gastroenterology 1990 98: 968–975
Chu CM, Liaw YF . Membrane staining for hepatitis B surface antigen on hepatocytes: a sensitive and specific marker of active viral replication in hepatitis B J Clin Pathol 1995 48: 470–473
Satoh O et al. Membrane structure of the hepatitis B virus surface antigen particle J Biochem (Tokyo) 2000 127: 543–550
Michalak TI et al. Antibody-directed complement-mediated cytotoxicity to hepatocytes from patients with chronic hepatitis B Clin Exp Immunol 1995 100: 227–232
Daeron M . Fc receptor biology Annu Rev Immunol 1997 15: 203–234
Machy P, Serre K, Leserman L . Class I-restricted presentation of exogenous antigen acquired by Fcgamma receptor-mediated endocytosis is regulated in dendritic cells Eur J Immunol 2000 30: 848–857
Mitchell DA, Nair SK, Gilboa E . Dendritic cell/macrophage precursors capture exogenous antigen for MHC class I presentation by dendritic cells (published erratum appears in Eur J Immunol 1998; 28: 3891) Eur J Immunol 1998 28: 1923–1933
Knight SC, Askonas BA, Macatonia SE . Dendritic cells as targets for cytotoxic T lymphocytes Adv Exp Med Biol 1997 417: 389–394
Parajuli P et al. Cytolysis of human dendritic cells by autologous lymphokine-activated killer cells: participation of both T cells and NK cells in the killing J Leukoc Biol 1999 65: 764–770
Cavazzana-Calvo M et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease Science 2000 28: 669–672
Wilson CB, Embree LJ, Schowalter D et al. Transient inhibition of CD28 and CD40 ligand interactions prolongs adenovirus-mediated transgene expression in the lung and facilitates expression after secondary vector administration J Virol 1998 72: 7542–7550
Halbert CL, Standaert TA, Wilson CB, Miller AD . Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure J Virol 1998 72: 9795–9805
Kagi D et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice Nature 1994 369: 31–37
Davis HL, Whalen RG, Demeneix BA . Direct gene transfer into skeletal muscle in vivo: factors affecting efficiency of transfer and stability of expression Hum Gene Ther 1993 4: 151–159
Krieg AM et al. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs Proc Natl Acad Sci USA 1998 95: 12631–12636
Davis HL et al. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle Hum Gene Ther 1993 4: 733–740
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem 1976 72: 248–254
Davis HL et al. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen J Immunol 1998 160: 870–876
Acknowledgements
We wish to thank Jocelyn Chandler, Brian Avery, George Condruit, and Chandimal Nicholas for their excellent technical support. This work was supported by a grant from the Medical Research Council of Canada to HLP. PJ Payette is a recipient of an Ontario Graduate Student Award and HL Davis is the recipient of an Ontario Ministry of Health Career Scientist Award.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Payette, P., Weeratna, R., McCluskie, M. et al. Immune-mediated destruction of transfected myocytes following DNA vaccination occurs via multiple mechanisms. Gene Ther 8, 1395–1400 (2001). https://doi.org/10.1038/sj.gt.3301534
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301534