Abstract
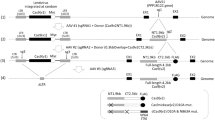
Recent work highlights the potential usefulness of MVM-based vectors as selective vehicles for cancer gene therapy (Dupont et al, Gene Therapy, 2000; 7: 790–796). To implement this strategy, however, it is necessary to develop optimized methods for producing high-titer, helper-free parvovirus stocks. Recombinants of MVMp (rMVMp) are currently generated by transiently co-transfecting permissive cell lines with a plasmid carrying the vector genome and a helper plasmid expressing the capsid genes (replaced with a foreign gene in the vector genome). The resulting stocks, however, are always heavily contaminated with replication-competent viruses (RCV), which precludes their use in vivo and particularly in gene therapy. In the present work we have developed a second-generation MVMp-based vector system specifically designed to reduce the probability of RCV generation by homologous recombination. We have constructed a new MVMp-based vector and a new helper genome with minimal sequence overlap and have used the degeneracy of the genetic code to further decrease vector–helper homology. In this system, the left homologous region was almost completely eliminated and the right sequence overlap was reduced to 74 nt with only 61% homology. We were thus able to substantially reduce (∼200 ×), but not completely eliminate, generation of contaminating viruses in medium-scale rMVMp preparations. Since the remaining sequence homology between the new vector and helper genomes is weak, our results suggest that contaminating viruses in this system are generated by nonhomologous recombination. It is important to note, unlike the autonomously replicating helper viruses produced from the first-generation vector/helper genomes, the contaminating viruses arising from the new packaging system cannot initiate secondary infection rounds (so they are not ‘replication-competent viruses’). Our findings have important implications for the design of new MVMp-based vectors and for the construction of trans-complementing packaging cell lines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Russell SJ et al. Transformation-dependent expression of interleukin genes delivered by a recombinant parvovirus J Virol 1992 66: 2821–2828
Dupont F et al. Use of an autonomous parvovirus vector for selective transfer of a foreign gene into transformed human cells of different tissue origins and its expression therein J Virol 1994 68: 1397–1406
Dupont F et al. Tumor-selective gene transduction and cell killing with an oncotropic autonomous parvovirus-based vector Gene Therapy 2000 7: 790–796
Russell SJ . Lymphokine gene therapy for cancer Immunol Today 1990 11: 196–200
Merchlinsky MJ et al. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice J Virol 1983 47: 227–232
Avalosse B et al. Method for concentrating and purifying recombinant autonomous parvovirus vectors designed for tumour-cell-targeted gene therapy J Virol Meth 1996 62: 179–183
El Bakkouri K et al. Amplification of MVM(p) vectors through serial infection of a new packaging cell line Tumor Targeting 1999 4: 210–217
Miller AD . Retrovirus packaging cells Hum Gene Ther 1990 1: 5–14
Allen JM et al. Identification and elimination of replication-competent adeno- associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production J Virol 1997 71: 6816–6822
Zhu J et al. Characterization of replication-competent adenovirus isolates from large-scale production of a recombinant adenoviral vector Hum Gene Ther 1999 10: 113–121
Maxwell IH et al. Recombinant LuIII autonomous parvovirus as a transient transducing vector for human cells Hum Gene Ther 1993 4: 441–450
Kestler J et al. cis Requirements for the efficient production of recombinant DNA vectors based on autonomous parvoviruses Hum Gene Ther 1999 10: 1619–1632
Subramani S, Rubnitz J . Recombination events after transient infection and stable integration of DNA into mouse cells Mol Cell Biol 1985 5: 659–666
Brandenburger A, Russell S . A novel packaging system for the generation of helper-free oncolytic MVM vector stocks Gene Therapy 1996 3: 927–931
Jongeneel CV et al. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus J Virol 1986 59: 564–573
Morgan WR, Ward DC . Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs J Virol 1986 60: 1170–1174
Tam P, Astell CR . Replication of minute virus of mice minigenomes: novel replication elements required for MVM DNA replication Virology 1993 193: 812–824
Tam P, Astell CR . Multiple cellular factors bind to cis-regulatory elements found inboard of the 5′ palindrome of minute virus of mice J Virol 1994 68: 2840–2848
Morgenstern JP, Land H . Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line Nucleic Acids Res 1990 18: 3587–3596
Faust EA, Ward DC . Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication J Virol 1979 32: 276
Shein HM, Enders JF . Multiplication and cytopathogenicity of simian vacuolating virus 40 in cultures of human tissue Proc Soc Exp Biol Med 1962 40: 495–500
Brandenburger A et al. Influence of sequence and size of DNA on packaging efficiency of parvovirus MVM-based vectors Hum Gene Ther 1999 10: 1229–1238
Naeger LK, Cater J, Pintel DJ . The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner J Virol 1990 64: 6166–6175
Girod A et al. Homologous and nonhomologous retroviral recombinations are both involved in the transfer by infectious particles of defective avian leukosis virus-derived transcomplementing genomes J Virol 1996 70: 5651–5657
Wang XS et al. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination J Virol 1998 72: 5472–5480
Hogan A, Faust EA . Short direct repeats mediate spontaneous high-frequency deletions in DNA of minute virus of mice Mol Cell Biol 1984 4: 2239–2242
Hogan A, Faust EA . Nonhomologous recombination in the parvovirus chromosome: role for a CTATTTCT motif Mol Cell Biol 1986 6: 3005–3009
Tullis GE, Burger LR, Pintel DJ . The minor capsid protein VP1 of the autonomous parvovirus minute virus of mice is dispensable for encapsidation of progeny single-stranded DNA but is required for infectivity J Virol 1993 67: 131–141
Haag A et al. Highly efficient transduction and expression of cytokine genes in human tumor cells by means of autonomous parvovirus vectors; generation of antitumor responses in recipient mice Hum Gene Ther 2000 11: 597–609
Inoue N, Russell DW . Packaging cells based on inducible gene amplification for the production of adeno-associated virus vectors J Virol 1998 72: 7024–7031
Sambrook J, Fritsch EJ, Maniatis T . Molecular Cloning: A Laboratory Manual Cold Spring Harbor Laboratory Press: New York 1989
Boissy R, Astell CR . An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5′-terminal palindrome of minute virus of mice Gene 1985 35: 179–185
Spegelaere P et al. Lack of a detectable effect of capsid proteins on the cell-dependent activity of parvovirus MVMp promoters Res Virol 1994 145: 5–12
Dupont F et al. Design of autonomous parvovirus vectors for tumor cell-targeted gene therapy: a functional comparison of replicative versus non-replicative MVMp-based vectors. (Submitted)
Acknowledgements
This work was supported by grants from the ‘Fonds National de la Recherche Scientifique’ (FNRS), the ‘Télévie’, ‘les Amis de Bordet’ and the Medic Foundation. We thank P Spegelaere for the gift of pSP116 and M El Boutaibi for constructing and characterizing pME3. We are grateful to B Van Vaerenbergh for critical reading of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dupont, F., Karim, A., Dumon, JC. et al. A novel MVMp-based vector system specifically designed to reduce the risk of replication-competent virus generation by homologous recombination. Gene Ther 8, 921–929 (2001). https://doi.org/10.1038/sj.gt.3301477
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301477