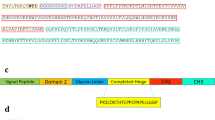
Abstract
Accumulating evidence suggests the involvement of TGF-β in the process of corneal opacity, which is one of the serious causes of visual loss. However, whether TGF-β is indeed critical for the pathogenesis remains unknown. We constructed an adenovirus expressing an entire ectodomain of the human type II TGF-β receptor fused to Fc portion of human IgG (AdTβ-ExR): this soluble receptor is secreted from AdTβ-ExR-infected cells, binds to TGF-β and inhibits TGF-β signaling. When AdTβ-ExR was injected into the femoral muscle of Balb/c mice, a high level of the soluble receptor protein (2.0–3.5 × 103 pM) was detectable in the serum and in the ocular fluid for at least 10 days. In the mice subjected to corneal injury with silver nitrate and to intramuscular injection with either saline or a control adenovirus expressing β-galactosidase (AdLacZ), corneal opacification composed of extracellular matrix (ECM) accumulation, of infiltration of neutrophils and monocytes/macrophages, and of angiogenesis were all induced. In contrast, they were markedly reduced in the mice injected with AdTβ-ExR. Immunohistochemical analysis revealed that TGF-β, fibronectin, macrophage chemoattractant protein-1, and vascular endothelial growth factor were densely stained in the edge of wounded cornea, but they were scarcely present in the injured-cornea of AdTβ-ExR-treated mice. Our results demonstrate that TGF-β indeed plays a critical role in the process of cornea opacification, and that adenovirus-mediated expression of a soluble TGF-β receptor can be therapeutically useful.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Wahl SM et al. TGF-β induces chemotaxis and growth factor production Proc Natl Acad Sci USA 1987 84: 5788–5792
Border WA, Noble NA . (1994) Transforming growth factor β in tissue fibrosis New Engl J Med 1994 331: 1286–1292
Moulin V . Growth factors in skin wound healing Eur J Cell Biol 1995 68: 1–7
Bikfalvi A . Significance of angiogenesis in tumour progression and metastasis Eur J Cancer 1995 31A: 1101–1104
Battegay EJ . Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects J Mol Med 1995 73: 333–346
Stavri GT et al. Basic firboblast growth factor upregulates the expression of vascular endothelial growth factor in vascular smooth muscle cells. Synergistic interaction with hypoxia Circulation 1995 92: 11–14
Sankar S et al. Modulation of transforming growth factor-β receptor levels on microvascular endothelial cells during in vitro angiogenesis J Clin Invest 1996 97: 1436–1446
Song QH et al. Transforming growth factor-β1 expression in cultured corneal fibroblasts in response to injury J Cell Biochem 2000 77: 186–199
Pasquale LR et al. Immunolocalization of TGF-β1, TGF-β2, and TGF-β3 in the anterior segment of the human eye Invest Ophthalmol Vis Sci 1993 34: 23–30
Wilson SE, He YG, Lloyd SA . EGF, EGF receptor, basic FGF, TGF β-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts Invest Ophthalmol Vis Sci 1992 33: 1756–1765
Nishida K et al. Immunohistochemical localization of transforming growth factor-β1, -β2, and -β3 latency-associated peptide in human cornea Invest Ophthalmol Vis Sci 1994 35: 3289–3294
Joyce NC, Zieske JD . Transforming growth factor-β receptor expression in human cornea Invest Ophthalmol Vis Sci 1997 38: 1922–1928
Hayashi K, Frangieh G, Wolf G, Kenyon KR . Expression of transforming growth factor-beta in wound healing of vitamin A-deficient rat corneas Invest Ophthalmol Vis Sci 1989 30: 239–247
Li DQ, Tseng SC . Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface J Cell Physiol 1995 163: 61–79
Shull MM et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease Nature 1992 359: 693–699
Kulkarni AB et al. Transforming growth factor-β null mutation in mice causes excessive inflammatory response and early death Proc Natl Acad Sci USA 1993 90: 770–774
Sanford LP et al. TGF-β2 knockout mice have multiple developmental defects that are nonoverlapped with other TGF-β phenotype Development 1997 124: 2659–2670
Ueno H et al. Quantitative analysis of repeat adenovirus-mediated gene transfer into injured canine femoral arteries Arterioscl Thromb Vasc Biol 1995 15: 2246–2253
Sakamoto T et al. Retinal functional change caused by an adenoviral-vector mediated gene transfer of LacZ gene Hum Gene Ther 1998 9: 789–799
Qi Z et al. Blockade of type β transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat Proc Natl Acad Sci USA 1999 96: 2345–2349
Abe M et al. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct Anal Biochem 1994 216: 276–284
Sonoda K et al. Inhibition of corneal inflammation by the topical use of Ras farnesyltransferase inhibitors: selective inhibition of macrophage localization Invest Ophthalmol Vis Sci 1998 39: 2245–2251
Yamamoto H, Ueno H, Ooshima A, Takeshita A . Adenovirus-mediated transfer of a truncated transforming growth factor-β (TGF-β) type II receptor completely and specifically abolishes diverse signaling by TGF-β in vascular wall cells in primary culture J Biol Chem 1996 271: 16253–16259
Kong HL et al. Regional suppression of tumor growth by in vivo transfer of a cDNA encoding a secreted form of the extracellular domain of the flt-1 vascular endothelial growth factor receptor Hum Gene Ther 1998 9: 823–833
Honda M et al. Adenovirus-mediated gene transfer of soluble flt-1 receptor inbihits the experimental subretinal neovascularization Gene Therapy 2000 7: 978–985
Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T . Release of TGF-β1 and VEGF in tears following photorefractive keratectomy Curr Eye Res 1997 16: 19–25
Sunderkötter C, Beil W, Roth J, Sorg C . Cellular events associated with inflammatory angiogenesis in the mouse cornea Am J Pathol 1991 138: 931–939
Reibman J et al. Transforming growth factor-β1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways Proc Natl Acad Sci USA 1991 88: 6805–6809
Takeshita A et al. TGF-β induces expression of monocyte chemoattractant JE/monocyte chemoattractant protein 1 via transcriptional factor AP-1 induced by protein kinase in osteoblastic cells J Immunol 1995 155: 419–426
Zheng MH et al. Gene expression of monocyte chemoattractant protein-1 in giant cell tumors of bone osteoclastoma: possible involvement in CD68+ macrophage-like cell migration J Cell Biochem 1998 70: 121–129
Kitamura M . Identification of an inhibitor targeting macrophage production of monocyte chemoattractant protein-1 as TGF-β1 J Immunol 1997 159: 1404–1411
Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C . Macrophages and angiogenesis J Leukocyte Biol 1994 55: 410–422
Pertovaara L et al. Vascular endothelial growth factor is induced in response to transforming growth factor-β in fibroblastic and epithelial cells J Biol Chem 1994 269: 6271–6274
Koochekpour S, Merzak A, Pilkington GJ . Vascular endothelial growth factor production is stimulated by gangliosides and TGF-β isoforms in human glioma cells in vitro Cancer Lett 1996 102: 209–215
Brogi E, Wu T, Namiki A, Isner JM . Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only Circulation 1994 90: 649–652
Streilein JW, Wilbanks GA, Taylor A, Cousins S . Eye-derived cytokines and the immunosuppressive intraocular microenvironment: a review Curr Eye Res 1992 11: (Suppl) 41–47
Chen KH, Harris DL, Joyce NC . TGF-β2 in aqueous humor suppresses S-phase entry in cultured corneal endothelial cells Invest Ophthalmol Vis Sci 1999 40: 2513–2519
Noisakran S, Campbell IL, Carr DJJ . Ectopic expression of DNA encoding IFN-a1 in the cornea protects mice from herpes simplex type 1-induced encephalitis J Immunol 1999 162: 4184–4190
Kuklin NA, Daheshia M, Chun S, Rouse BT . Immunomodulation by mucosal gene transfer using TGF-β DNA J Clin Invest 1998 102: 438–444
Tsubota K et al. Adenovirus-mediated gene transfer to the ocular surface epithelium Exp Eye Res 1998 67: 531–538
Wilson JM . Adenoviruses as gene-delivery vehicles New Engl J Med 1996 334: 1185–1187
Kenyon BM et al. A model of angiogenesis in the mouse corneas Invest Ophthalmol Vis Sci 1996 37: 1625–1632
Benelli U, Ross JR, Nardi M, Klintworth GK . Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A Invest Ophthalmol Vis Sci 1997 38: 274–282
Leenen PJM et al. Markers of mouse macrophage development detected by monoclonal antibodies J Immunol Methods 1994 174: 5–19
Haidl ID, Jefferies WA . The macrophage cell surface glycoprotein F4/80 is a highly glycosylated proteoglycan Eur J Immunol 1996 26: 1139–1146
Lewinsohn DM, Bargatze RF, Butcher EC . Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes J Immunol 1987 138: 4313–4321
Olofsson A et al. Transforming growth factor-β1, -β2, and -β3 secreted by a human glioblastoma cell line. Identification of small and different forms of large latent complexes J Biol Chem 1992 267: 19482–19488
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from Ministry of Education, Science and Culture of the Japanese Government (to TS and HU), and by the grants from Japan National Society for the Prevention of Blindness (Tokyo, Japan), from the Fukuoka Anti-Cancer Association (Fukuoka, Japan), from Kaibara Morikazu Medical Science Promotion Foundation (Fukuoka, Japan), from The Casio Science Promotion Foundation (Tokyo, Japan) (to TS), and from Takeda Medical Research Foundation and from Tokyo Biochemical Society (HU). We also thanks Drs H Yamashita and K Miyazono for their kind gift of TGF-β antibodies and Drs H Sanui and M Uehara for their financial support.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakamoto, T., Ueno, H., Sonoda, K. et al. Blockade of TGF-β by in vivo gene transfer of a soluble TGF-β type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther 7, 1915–1924 (2000). https://doi.org/10.1038/sj.gt.3301320
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301320
Keywords
This article is cited by
-
TGFβ pathobiology in the eye
Laboratory Investigation (2006)
-
Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-β type II receptor gene
Cancer Gene Therapy (2006)
-
Nerve growth factor modulates in vitro the expression and release of TGF-β1 by amniotic membrane
Graefe's Archive for Clinical and Experimental Ophthalmology (2006)
-
EDB fibronectin and angiogenesis – a novel mechanistic pathway
Angiogenesis (2005)
-
Inhibition of transforming growth factor β decreases pancreatic fibrosis and protects the pancreas against chronic injury in mice
Laboratory Investigation (2004)