Abstract
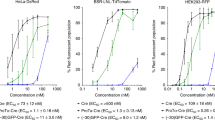
Condensing peptide–DNA complexes have great potential as nonviral agents for gene delivery. To date, however, such complexes have given transfection activities greatly inferior to adenovirus and somewhat inferior to cationic lipid–DNA complexes, even for cell lines and primary cells in vitro. We report here the identification of a novel condensing peptide, CL22, which forms DNA complexes that efficiently transfect many cell lines, as well as primary dendritic and endothelial cells. We report studies with sequence and structure variants that define some properties of the peptide that contribute to efficient transfection. We demonstrate that the superior transfection activity of CL22 compared with other DNA condensing peptides is conferred at a step after uptake of the complexes into cells. We show that CL22–DNA complexes have transfection activity that is at least equivalent to the best available nonviral agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Treco DA, Selden RF . Non-viral gene therapy Mol Med Today 1995 1: 314–321
Cristiano RJ . Targeted, non-viral gene delivery for cancer gene therapy Front Biosci 1998 3: D1161–D1170
Mahato RI, Takakura Y, Hashida M . Non-viral vectors for in vivo gene delivery: physicochemical and pharmacokinetic considerations Crit Rev Ther Drug Carrier Syst 1997 14: 133–172
Wu CH, Wilson JM, Wu GY . Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo J Biol Chem 1989 264: 16985–16987
Perales JC, Ferkol T, Molas M, Hanson RW . An evaluation of receptor-mediated gene transfer using synthetic DNA–ligand complexes Eur J Biochem 1994 226: 255–266
Wadhwa MS et al. Peptide-mediated gene delivery: influence of peptide structure on gene expression Bioconjug Chem 1997 8: 81–88
Gottschalk S et al. A novel DNA–peptide complex for efficient gene transfer and expression in mammalian cells Gene Therapy 1996 3: 48–57
Emi N, Kidoaki S, Yoshikawa K, Saito H . Gene transfer mediated by polyarginine requires a formation of big carrier-complex of DNA aggregate Biochem Biophys Res Commun 1997 231: 421–424
Boussif O et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine Proc Natl Acad Sci USA 1995 92: 7297–7301
Chemin I et al. Liver-directed gene transfer: a linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo J Viral Hepat 1998 5: 369–375
Goula D et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung Gene Therapy 1998 5: 1291–1295
Ferrari S et al. ExGen 500 is an efficient vector for gene delivery to lung epithelial cells in vitro and in vivo Gene Therapy 1997 4: 1100–1106
Haensler J, Szoka FC Jr . Polyamidoamine cascade polymers mediate efficient transfection of cells in culture Bioconjug Chem 1993 4: 372–379
Tang MX, Redemann CT, Szoka FC Jr . In vitro gene delivery by degraded polyamidoamine dendrimers Bioconjug Chem 1996 7: 703–714
Felgner PL et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure Proc Natl Acad Sci USA 1987 84: 7413–7417
Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J . Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA Proc Natl Acad Sci USA 1989 86: 6982–6986
Lasic DD, Templeton NS . Liposomes in gene therapy Adv Drug Del Rev 1996 20: 221–226
Lemieux GA, Bertozzi CR . Chemoselective ligation reactions with proteins, oligosaccharides and cells Trends Biotechnol 1998 16: 506–513
Keilová H . On the specificity and inhibition of cathepsins D and B. In: Barrett AJ, Dingle JT (eds) Tissue Proteinases North Holland Publishing: Amsterdam 1971 pp 45–68
Kirschke H, Barrett AJ . Chemistry of lysosomal proteases. In: Glaumann H, Ballard FJ (eds) Lysosomes: Their Role in Protein Breakdown Academic Press: London 1987 193–238
Brett SJ et al. Human T cell recognition of influenza A nucleoprotein. Specificity and genetic restriction of immunodominant T helper cell epitopes J Immunol 1991 147: 984–991
Wu GY, Wu CH . Receptor-mediated in vitro gene transformation by a soluble DNA carrier system (published erratum appears in J Biol Chem 1988; 263: 588) J Biol Chem 1987 262: 4429–4432
Avrameas A et al. Polyreactive anti-DNA monoclonal antibodies and a derived peptide as vectors for the intracytoplasmic and intranuclear translocation of macromolecules Proc Natl Acad Sci USA 1998 95: 5601–5606
Zhang F et al. A transfecting peptide derived from adenovirus fiber protein Gene Therapy 1999 6: 171–181
Thatcher DR, Haines A, Phillips R . Peptide-mediated gene delivery. In: Kabanov AV, Felgner PL, Seymour LW (eds) Self-Assembling Complexes for Gene Delivery: From Laboratory to Clinical Trial John Wiley: Chichester 1998 pp 331–350
Adami RC et al. Stability of peptide-condensed plasmid DNA formulations J Pharm Sci 1998 87: 678–683
Dash PR, Toncheva V, Schacht E, Seymour LW . Synthetic polymers for vectorial delivery of DNA: characteristics of polymer DNA complexes by photon correlation spectroscopy and stability to nuclease degradation and disruption by polyanions in vitro J Control Rel 1997 48: 269–276
Ogris M et al. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells Gene Therapy 1998 5: 1425–1433
Dash PR et al. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery Gene Therapy 1999 6: 643–650
Irvine AS et al. Efficient nonviral transfection of dendritic cells and their use for in vivo immunization Nat Biotechnol 2000 18: 1273–1278
Arthur JF et al. A comparison of gene transfer methods in human dendritic cells Cancer Gene Ther 1997 4: 17–25
Philip R et al. Transgene expression in dendritic cells to induce antigen-specific cytotoxic T cells in healthy donors Cancer Gene Ther 1998 5: 236–246
Hanke T et al. DNA multi-CTL epitope vaccines for HIV and Plasmodium falciparum: immunogenicity in mice Vaccine 1998 16: 426–435
Romani N et al. Proliferating dendritic cell progenitors in human blood J Exp Med 1994 180: 83–93
Felgner PL et al. Nomenclature for synthetic gene delivery systems (editorial) Hum Gene Ther 1997 8: 511–512
Acknowledgements
We thank Dr Tim Booth (currently at CFIA, National Centre for Foreign Animal Disease,Winnipeg, Canada) and Dr Svetla Stoilova (Leeds University, UK) for Cryo-Electron microscopy. We would also like to thank Professor Nick Price and Dr Sharon Kelly at BBSRC Scottish Circular Dichroism Facility for their generous help and advice.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Haines, A., Irvine, A., Mountain, A. et al. CL22 – a novel cationic peptide for efficient transfection of mammalian cells. Gene Ther 8, 99–110 (2001). https://doi.org/10.1038/sj.gt.3301314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301314
Keywords
This article is cited by
-
Phase I/II trial of a dendritic cell vaccine transfected with DNA encoding melan A and gp100 for patients with metastatic melanoma
Gene Therapy (2011)
-
Novel cGMP liposomal vectors mediate efficient gene transfer
Cancer Gene Therapy (2003)