Abstract
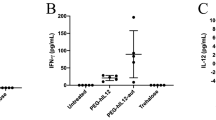
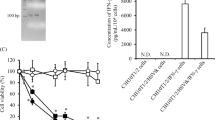
The possibility of achieving multiple systemic expression of human interferon-β in mice upon repeated intravenous administration of cationic liposome–DNA complex (lipoplex) was investigated. Lipoplexes containing the pentammonio lipid pcTG90 were first optimized by selecting the most efficient ratio of pcTG90 to phosphatidylethanolamine (DOPE) and the N/P ratio of cationic lipid nitrogen to DNA phosphate. Highest levels and reproducibility of gene expression were obtained using pcTG90/DOPE (1:2) liposomes complexed with the IFNB1 gene containing plasmid pTG14169 at a N/P ratio of 10. Following lipoplex administration, an early but transient human interferon-β expression in serum was observed. Importantly, repeated systemic gene expression could be achieved upon re-administration with a minimal time interval of 14 days between two injections. For an interval period of 6 days, subsequent gene expression was inhibited by a first administration of lipoplexes containing either a luciferase reporter gene plasmid or an empty plasmid, but was not inhibited when free (non-complexed) plasmid pTG14169 was first injected. Multiple injections of pcTG90-lipoplex performed once every other month resulted in three subsequent peaks of systemic IFNB1 gene expression in mice. In conclusion, our study demonstrates the feasibility of expanding the therapeutic window of a cytokine using repetitive intravenous administration of lipoplex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Felgner PL et al. Nomenclature for synthetic gene delivery systems Hum Gene Ther 1997 8: 511–512
Crystal RG . Transfer of genes to humans: early lessons and obstacles to success Science 1995 270: 404–410
Ledley FD . Nonviral gene therapy: the promise of genes as pharmaceutical products Hum Gene Ther 1995 6: 1129–1144
Gao X, Huang L . Cationic liposome-mediated gene transfer Gene Therapy 1995 2: 710–722
Einhorn S, Grander D . Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res 1996 16: 275–281
Salmon P et al. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers J Interferon Cytokine Res 1996 16: 759–764
Quesada JR . Clinical toxicity of interferons in cancer patients: a review J Clin Oncol 1986 4: 234–243
Song YK, Liu F, Chu S, Liu D . Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration Hum Gene Ther 1997 8: 1585–1594
Hong K, Zheng W, Baker A, Papahadjopoulos D . Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery FEBS Lett 1997 400: 233–237
Liu F, Qi H, Huang L, Liu D . Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration Gene Therapy 1997 4: 517–523
Liu Y et al. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery Nat Biotechnol 1997 15: 167–173
Sternberg B . Ultrastructural morphology of cationic liposome-DNA complexes for gene therapy. In: Lasic DD, Papahadjopoulos D (eds) Medical Applications of Liposomes Elsevier: Amsterdam 1998 395–427
Smith JG . Characterization and in vivo testing of a heterogeneous cationic lipid-DNA formulation Pharm Res 1998 15: 1356–1363
Song YK, Liu D . Free liposomes enhance the transfection activity of DNA/lipid complexes in vivo by intravenous administration Biochim Biophys Acta 1998 1372: 141–150
Song X-Y et al. Plasmid DNA encoding transforming growth factor-β1 suppresses chronic disease in a streptococcal cell wall-induced arthritis model J Clin Invest 1998 101: 2615–2621
Vehar GA, Lawn RM, Tuddenham EGD, Wood WI . Factor VIII and factor V: biochemistry and pathophysiology. In: Albertini A, Lenfant CL, Mannucci PM, Sixma JJ (eds) Biotechnology of Plasma Proteins Karger: Basel 1991 2155–2170
Kreiss P, Bettan M, Crouzet J, Scherman D . Erythropoietin secretion and physiological effect in mouse after intramuscular plasmid DNA electrotransfer J Gene Med 1999 1: 245–250
Blezinger P et al. Systemic inhibition of tumor growth and tumor metastases by intramuscular administration of the endostatin gene Nat Biotechnol 1999 17: 343–348
Li S et al. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors Am J Physiol 1999 276: L796–L804
Meyer O et al. Cationic liposomes coated with polyethylene glycol as carriers for oligonucleotides J Biol Chem 1998 273: 15621–15627
Nazih A et al. Synthesis and stability of the new pentammonio lipid pcTG90, a gene transfer agent Tetrahedron Lett 1999 40: 8089–8091
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning: A Laboratory Manual Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY 1989 vol. 1: pp 21–24
Bartlett GR . Phosphorus assay in column chromatography J Biol Chem 1959 234: 466–469
Schughart K et al. Effect of liposome-encapsulated clodronate pretreatment on synthetic vector-mediated gene expression in mice Gene Therapy 1999 6: 448–453
Acknowledgements
Part of this work was supported by the AFLM (Association Française de Lutte contre la Mucovisidose) and the AFM (Association Française contre les Myopathies). We would like to thank D Elmlinger, O Roch, C Mougin, D Ali-Hadji, F Augé, G Amann for their valuable technical assistance, Dr B Cavallini, I Renardet and C Brua for plasmid preparations, Drs N Silvestre and U Rasmussen for plasmid constructions, Drs D Heissler and A Nazih (Université Louis Pasteur, Strasbourg) for providing the cationic lipid pcTG90 and M Autem for help with statistical analyses. We are grateful to Drs M Courtney and S Braun for critical review of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meyer, O., Schughart, K., Pavirani, A. et al. Multiple systemic expression of human interferon-β in mice can be achieved upon repeated administration of optimized pcTG90-lipoplex. Gene Ther 7, 1606–1611 (2000). https://doi.org/10.1038/sj.gt.3301289
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301289
Keywords
This article is cited by
-
Interferon-β lipofection II. Mechanisms involved in cell death and bystander effect induced by cationic lipid-mediated interferon-β gene transfer to human tumor cells
Cancer Gene Therapy (2012)
-
Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord
Gene Therapy (2003)