Abstract
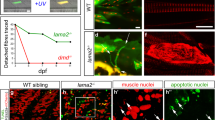
Laminin-2 is part of the basement membrane of the skeletal muscle fibers. The laminin α2 chain is absent or drastically reduced in a subgroup of congenital muscular dystrophy patients, and in the severely affected dystrophic dy/dy mouse. We previously reported that heterogenous primary mouse muscle cell cultures conferred laminin α2 chain expression in dy/dy mice muscles upon cell transplantation. In the present study we investigated whether pure myoblast cell lines were able to confer laminin α2 chain expression in vivo. We observed that: (1) xenotransplantation of non-immortalized human myoblast in SCID mouse muscles allows human laminin α2 chain expression; (2) allotransplantation of the permanent G8 mouse myoblast cell line in dy/dy muscles allows the expression of the murine laminin α2 chain; and (3) allotransplantation of the D7 dystrophic dy/dy cell line allows the formation of new and hybrid muscle fibers in dy/dy muscle in the absence of laminin α2 chain expression. We conclude that normal myoblasts are able to restore the expression of an extracellular skeletal muscle protein and that the absence of laminin-2 does not prevent transplanted muscle cells from participating in the formation of myofibers. Myoblasts are, therefore, attractive tools for further exploration of gene complementation strategies in the animal models of congenital muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Leivo I, Engvall E . Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development Proc Natl Acad Sci USA 1988 85: 1544–1548
Ehrig K et al. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein Proc Natl Acad Sci USA 1990 87: 3264–3268
Vuolteenaho R et al. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues J Cell Biol 1994 124: 381–394
Schuler F, Sorokin LM . Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo J Cell Sci 1995 108: 3795–3805
Sewry CA et al. Diagnosis of merosin (laminin-2) deficient congenital muscular dystrophy by skin biopsy Lancet 1996 347: 582–584
Burgeson RE et al. A new nomenclature for the laminins Matrix Biol 1994 14: 209–211
Engvall E, Wewer UM . Domains of laminin J Cell Biochem 1996 61: 493–501
Campbell KP . Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage Cell 1995 80: 675–679
Patton BL, Miner JH, Chiu AI, Sanes JR . Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice J Cell Biol 1997 139: 1507–1521
Vachon PH et al. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival J Cell Biol 1996 134: 1483–1498
Kuang W, Xu H, Vachon PH, Engvall E . Disruption of the lama2 gene in embryonic stem cells; laminin α2 is necessary for sustenance of mature muscle cells Exp Cell Res 1998 241: 117–125
Tomé FMS et al. Congenital muscular dystrophy with merosin deficiency CR Acad Sci Paris 1994 317: 351–357
Helbling-Leclerc A et al. Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy Nat Genet 1995 11: 216–218
Nissinen M et al. Substitution of a conserved cysteine-996 in a cysteine-rich motif of the laminin α2 chain in congenital muscular dystrophy with partial deficiency of the protein Am J Hum Genet 1996 58: 1177–1184
Zhang X, Vuolteenaho R, Tryggvason K . Structure of the human laminin alpha2-chain gene (LAMA2), which is affected in congenital muscular dystrophy J Biol Chem 1996 271: 27664–27669
Michelson AM, Russell ES, Harman PJ . Dystrophia muscularis: a hereditary primary myopathy in the house mouse Proc Natl Acad Sci USA 1955 41: 1079–1084
Arahata A et al. Laminin in animal models for muscular dystrophy. Defect of laminin M in skeletal and cardiac muscles and peripheral nerve of the homozygous dystrophic dy/dy mice Proc Jpn Acad 1993 69: 259–264
Sunada Y et al. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus J Biol Chem 1994 269: 13729–13732
Xu H et al. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse Proc Natl Acad Sci USA 1994 91: 5572–5576
Meier H, Southard JL . Muscular dystrophy in the mouse caused by an allele at the dylocus Life Sci 1970 9: 137–144
Xu H, Wu XR, Wewer UM, Engvall E . Murine muscular dystrophy caused by a mutation in the laminin α2 (Lama2) gene Nat Genet 1994 8: 297–302
Sunada Y et al. Identification of a novel mutant transcript of laminin α2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J/dy2J mice Hum Mol Genet 1995 4: 1055–1061
Miyagoe Y et al. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy FEBS Lett 1997 415: 33–39
Kuang W et al. Merosin-deficient congenital muscular dystrophy; partial genetic correction in two mouse models J Clin Invest 1998 102: 844–852
Vilquin JT et al. Partial laminin α2 chain restoration in laminin α2 chain-deficient dy/dy mouse by primary muscle cell culture transplantation J Cell Biol 1996 133: 185–197
Haider SR, Wang W, Kaufman SJ . SV40 T antigen inhibits expression of MyoD and myogenin, up-regulates Myf-5, but does not affect early expression of desmin or α7 integrin during muscle development Exp Cell Res 1994 210: 278–286
Tedesco D, Caruso M, Fischer-Fantuzzi L, Vesco C . The inhibition of cultured myoblast differentiation by the simian virus 40 large T antigen occurs after myogenin expression and Rb up-regulation and is not exerted by transformation-competent cytoplasmic mutants J Virol 1995 69: 6947–6957
Christian CN, Nelson PG, Peacock J, Nirenberg M . Synapse formation between two clonal cell lines Science 1977 196: 995–998
Yaffe D, Saxel O . Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle Nature 1977 270: 725–727
Tremblay JP et al. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy Cell Transplant 1993 2: 99–112
Huard J et al. High efficacy of muscle regeneration following human myoblast transplantation in SCID mice J Clin Invest 1994 93: 586–599
Adam M, Ramesh N, Miller DA, Osborne WRA . Internal initiation of translation in retroviral vectors carrying picornavirus 5′ nontranslated regions J Virol 1991 65: 4985–4990
Kinoshita I et al. Very efficient myoblast allotransplantation in mice under FK506 immunosuppression Mus Nerve 1994 17: 1407–1415
Kinoshita I et al. Transplantation of myoblasts from a transgenic mouse overexpressing dystrophin produced only a relatively small increase of dystrophin positive membrane Mus Nerve 1997 21: 91–103
Berndt A, Kosmehl H, Katenkamp D, Tauchmann V . Appearance of the myofibroblastic phenotype in Dupuytren’s disease is associated with a fibronectin, laminin, collagen type IV and tenascin extracellular matrix Pathobiology 1994 62: 55–58
Hagg T, Portera-Cailliau C, Jucker M, Engvall E . Laminins of the adult mammalian CNS; laminin-α2 (merosin M−) chain immunoreactivity is associated with neuronal processes Brain Res 1997 764: 17–27
Partridge TA et al. Conversion of mdx myofibres from dystrophin-negative to-positive by injection of normal myoblasts Nature 1989 337: 176–179
Morgan JE, Hoffman EP, Partridge TA . Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse J Cell Biol 1990 111: 2437–2449
Vilquin JT et al. Successful histocompatible myoblast transplantation in dystrophin-deficient mdx mouse despite the production of antibodies against dystrophin J Cell Biol 1995 131: 975–988
Guérette B et al. Immunosuppression with monoclonal antibodies and CTLA4-Ig after myoblast transplantation in mice Transplantation 1996 62: 962–967
Guérette B et al. Prevention by anti-LFA-1 of acute myoblast death following transplantation J Immunol 1997 159: 2522–2531
Qu Z et al. Development of approaches to improve cell survival in myoblast transfer therapy J Cell Biol 1998 142: 1257–1267
Floyd SS et al. Ex vivo gene transfer using adenovirus-mediated full-length dystrophin delivery to dystrophic muscles Gene Therapy 1998 5: 19–30
Moisset PA et al. Successful transplantation of genetically corrected DMD myoblasts following ex vivo transduction with the dystrophin minigene Biochem Biophys Res Com Mun 1998 247: 94–99
Merly F et al. Anti-inflammatory effect of transforming growth factor-betal in myoblast transplantation Transplantation 1998 65: 793–799
Kinoshita I, Vilquin JT, Tremblay JP . Pretreatment of myoblast cultures with basic fibroblast growth factor increases the efficacy of their transplantation in mdx mice Mus Nerve 1995 18: 834–841
Kinoshita I, Vilquin JT, Roy R, Tremblay JP . Successive injections in mdx mice of myoblasts grown with a high concentration of bFGF Neuromusc Dis 1996 6: 187–193
Ito H, Hallauer PL, Hastings KE, Tremblay JP . Prior culture with concanavalin A increases intramuscular migration of transplanted myoblasts Mus Nerve 1998 21: 291–297
Webster C et al. Isolation of human myoblasts with the fluorescence-activated cell sorter Exp Cell Res 1988 174: 252–265
Wernig A et al. Formation of new muscle fibres and tumours after injection of cultured myogenic cells J Neurocytol 1991 20: 982–997
Koelle GB, Friedenwald JS . A histochemical method for localizing cholinesterase activity Proc Soc Exp Biol Med 1949 70: 617–622
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vilquin, JT., Guérette, B., Puymirat, J. et al. Myoblast transplantations lead to the expression of the laminin α2 chain in normal and dystrophic (dy/dy) mouse muscles. Gene Ther 6, 792–800 (1999). https://doi.org/10.1038/sj.gt.3300889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3300889
Keywords
This article is cited by
-
Congenital muscular dystrophy-associated inflammatory chemokines provide axes for effective recruitment of therapeutic adult stem cell into muscles
Stem Cell Research & Therapy (2020)
-
Advances in genetic hearing loss: CIB2 gene
European Archives of Oto-Rhino-Laryngology (2017)
-
Myoblasts and Embryonic Stem Cells Differentially Engraft in a Mouse Model of Genetic Dilated Cardiomyopathy
Molecular Therapy (2013)
-
Skeletal muscle laminin and MDC1A: pathogenesis and treatment strategies
Skeletal Muscle (2011)
-
Normal growth and regenerating ability of myoblasts from unaffected muscles of facioscapulohumeral muscular dystrophy patients
Gene Therapy (2005)