Abstract
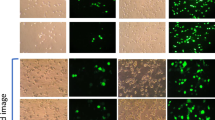
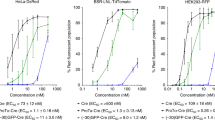
The inhibition effect of serum on the transfection efficiency of cationic liposome–DNA complexes (lipoplexes) is a major obstacle to the application of this gene delivery vector both in vitro and in vivo. The size of the lipoplexes, as they are presented to targeted cells, is found to be the major determinant of their effectiveness in transfection. The transfection efficiency and the cell association and uptake of lipoplexes with CHO cells was found to increase with increasing lipoplex size. The influence on the transfec- tion efficiency of lipoplexes by their cationic lipid:DNA ratios, types of liposomes, incubation time in polyanion containing media, and time of serum addition, are mediated mainly through size. Lipoplexes at a 2:1 charge ratio grow in size in media containing polyanions. The size growth may be arrested by adding serum to the incubation media. By using large lipoplexes, especially those made from multilamellar vesicles, the serum inhibition effect may be overcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Felgner PL et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure Proc Natl Acad Sci USA 1987 84: 7413–7417
Felgner PL et al. Nomenclature for synthetic gene delivery systems Hum Gene Ther 1997 8: 511–512
McLachlan G et al. Evaluation in vitro and in vivo of cationic liposome-expression construct complexes for cystic fibrosis gene therapy Gene Therapy 1995 2: 614–622
Crystal RG et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis Nat Genet 1994 8: 42–51
Felgner PL et al. Improved cationic lipid formulations for in vivo gene therapy Ann NY Acad Sci 1995 772: 126–139
Hui SW et al. The role of helper lipids in cationic liposome-mediated gene transfer Biophys J 1996 71: 590–599
Eastman SJ et al. Biophysical characterization of cationic lipid:DNA complexes Biochim Biophys Acta 1997 1325: 41–61
Sternberg B, Sorgi FL, Huang L . New structures in complex formation between DNA and cationic liposomes visualized by freeze-fracture electron microscopy FEBS Lett 1994 356: 361–366
Wheeler CJ et al. Converting an alcohol to an amine in a cationic lipid dramatically alters the co-lipid requirement, cellular transfection activity and the ultrastructure of DNA–cytofectin complexes Biochim Biophys Acta 1996 1280: 1–11
Xu Y, Szoka FC . Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection Biochemistry 1996 35: 5616–5623
Radler JO, Koltover I, Salditt T, Safinya CR . Structure of DNA–cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes Science 1997 275: 810–814
Duzgenes N, Goldstein JA, Friend DS, Felgner PL . Fusion of liposomes containing a novel cationic lipid, N-[2,3-(Dioleyloxy) propyl]-N, N, N-trimethylammonium: induction of multivalent anions and asymmetric fusion with acidic phospholipid vesicles Biochemistry 1989 28: 9179–9184
Wrobel I, Collins D . Fusion of cationic liposomes with mammalian cells occurs after endocytosis Biochim Biophys Acta 1995 1235: 296–304
Hui SW, Zhao YL . The DNA uptake mechanism of transfection mediated by cationic liposomes Zoological Studies 1995 34: 73–75
van der Woude I et al. Parameters influencing the introduction of plasmid DNA into cells by the use of synthetic amphiphiles as a carrier system Biochim Biophys Acta 1995 1240: 34–40
Staggs DR, Burton DW, Deftos LJ . Importance of liposome complexing volume in transfection optimization BioTechniques 1996 21: 792–795
Felgner JH et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations J Biol Chem 1994 269: 2550–2561
Farhood H, Serbina N, Huang L . The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer Biochim Biophys Acta 1995 1235: 289–295
Gao X, Huang L . Cationic liposome-mediated gene transfer Gene Therapy 1995 2: 710–722
Friend DS, Papahadjopoulos D, Debs RJ . Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes Biochim Biophys Acta 1996 1278: 41–50
Farhood H, Bottega R, Epand RM, Huang L . Effect of cationic cholesterol derivatives on gene transfer and protein kinase C activity Biochim Biophys Acta 1992 1111: 239–246
Rose JK, Buonocore L, Whitt MA . A new cationic liposome reagent yielding nearly quantitative transfection of animal cells BioTechniques 1991 10: 520–525
Bonte F, Juliano RL . Interactions of liposomes with serum proteins Chem Phys Lipids 1986 40: 359–372
Yang JP, Huang L . Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA Gene Therapy 1997 4: 950–960
Lewis JG et al. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA Proc Natl Acad Sci USA 1996 93: 3176–3181
Stegman T, Legendre JY . Gene transfer mediated by cationic lipids: lack of correlation between lipid mixing and transfection Biochim Biophys Acta 1997 1325: 71–79
Akao T et al. Correlation between physicochemical characteristics of synthetic cationic amphiphiles and their DNA transfection ability Bull Chem Soc Jpn 1991 64: 3677–3681
Zhang YP et al. Self-assembling DNA–lipid particles for gene transfer Pharm Res 1997 14: 190–196
Jaaskelainen I, Monkkonen J, Urtti A . Oligonucleotide-cationic liposome interactions. A physicochemical study Biochim Biophys Acta 1994 1195: 115–123
Boukhnikachvili T et al. Structure of in-serum transfecting DNA–cationic lipid complexes FEBS Lett 1997 409: 188–194
Ishikawa Y, Muramatsu N, Ohshima H, Kondo T . Effect of particle size on phagocytosis of latex particles by guinea-pig polymorphonuclear leucocytes J Biomats Sci Polymer Ed 1991 2: 53–60
Seymour L, Schacht E, Duncan R . The effect of size of polystyrene particles on their retention within the rat peritoneal compartment, and on their interaction with rat peritoneal macrophages in vitro Cell Biol Intl Rep 1991 15: 277–286
McLean JW et al. Organ-specific endothelial cell uptake of cationic liposome–DNA complexes in mice Am J Physiol 1997 273: H387–H404
Boussif O, Zanta MA, Behr JP . Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold Gene Therapy 1996 3: 1074–1080
Takeuchi K et al. Effect of zeta potential of cationic liposomes containing cholesterol derivatives on gene transfection FEBS Lett 1996 397: 207–209
Yang JP, Huang L . Time-dependent maturation of cationic liposome–DNA complexes for serum resistance Gene Therapy 1998 5: 380–387
Lee RJ, Huang L . Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer J Biol Chem 1996 271: 8481–8487
Machy P, Leserman LD . Small liposomes are better than large liposomes for specific drug delivery in vitro Biochim Biophys Acta 1983 730: 313–320
Langer M, Isac T, Hui SW . Interaction of free fatty acids with phospholipid bilayers Biochim Biophys Acta 1995 1236: 73–80
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ross, P., Hui, S. Lipoplex size is a major determinant of in vitro lipofection efficiency. Gene Ther 6, 651–659 (1999). https://doi.org/10.1038/sj.gt.3300863
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3300863
Keywords
This article is cited by
-
Tracking the Evolution of Transiently Transfected Individual Cells in a Microfluidic Platform
Scientific Reports (2018)
-
Lipid-based Transfection Reagents Exhibit Cryo-induced Increase in Transfection Efficiency
Molecular Therapy - Nucleic Acids (2016)
-
Generation of Transgenic Porcine Fibroblast Cell Lines Using Nanomagnetic Gene Delivery Vectors
Molecular Biotechnology (2016)
-
A Novel Formulation Based on 2,3-Di(tetradecyloxy)propan-1-amine Cationic Lipid Combined with Polysorbate 80 for Efficient Gene Delivery to the Retina
Pharmaceutical Research (2014)
-
Bioresponsive Deciduous-Charge Amphiphiles for Liposomal Delivery of DNA and siRNA
Pharmaceutical Research (2013)