Abstract
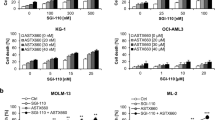
Internal tandem duplications (ITDs) of fms-like tyrosine kinase 3 (FLT3) receptor play an important role in the pathogenesis of acute myeloid leukemia (AML) and represent an attractive therapeutic target. ABT-869 has demonstrated potent effects in AML cells with FLT3-ITDs. Here, we provide further evidence that ABT-869 treatment significantly downregulates cyclins D and E but increases the expression of p21 and p27. ABT-869 induces apoptosis through downregulation of Bcl-xL and upregulation of BAK, BID and BAD. We also evaluate the combinations of ABT-869 and chemotherapy. ABT-869 demonstrates significant sequence-dependent synergism with cytarabine and doxorubicin in cell lines and primary leukemia samples. The optimal combination was validated in MV4-11 xenografts. Low-density array analysis revealed the synergistic interaction involved in downregulation of cell cycle and mitogen-activated protein kinase pathway genes. CCND1 and c-Mos were the most significantly inhibited targets on both transcriptional and translational levels. Treatment with short hairpin RNAs targeting either CCND1 or c-Mos further sensitized MV4-11 cells to ABT-869. These findings suggest that specific pathway genes were further targeted by adding chemotherapy and support the rationale of combination therapy. Thus, a clinical trial using sequence-dependent combination therapy with ABT-869 in AML is warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001; 97: 2434–2439.
Gilliland DG, Griffin JD . The roles of FLT3 in hematopoiesis and leukemia. Blood 2002; 100: 1532–1542.
Levis M, Small D . FLT3: ITDoes matter in leukemia. Leukemia 2003; 17: 1738–1752.
Stirewalt DL, Radich JP . The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 2003; 3: 650–665.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000; 19: 624–631.
Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000; 96: 3907–3914.
Zheng R, Friedman AD, Levis M, Li L, Weir EG, Small D . Internal tandem duplication mutation of FLT3 blocks myeloid differentiation through suppression of C/EBPalpha expression. Blood 2004; 103: 1883–1890.
Takahashi S, McConnell MJ, Harigae H, Kaku M, Sasaki T, Melnick AM et al. The Flt3 internal tandem duplication mutant inhibits the function of transcriptional repressors by blocking interactions with SMRT. Blood 2004; 103: 4650–4658.
Takahashi S, Harigae H, Kameoka J, Sasaki T, Kaku M . AML1B transcriptional repressor function is impaired by the Flt3-internal tandem duplication. Br J Haematol 2005; 130: 428–436.
Schwable J, Choudhary C, Thiede C, Tickenbrock L, Sargin B, Steur C et al. RGS2 is an important target gene of Flt3-ITD mutations in AML and functions in myeloid differentiation and leukemic transformation. Blood 2005; 105: 2107–2114.
Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res 2005; 65: 9643–9650.
Scheijen B, Ngo HT, Kang H, Griffin JD . FLT3 receptors with internal tandem duplications promote cell viability and proliferation by signaling through Foxo proteins. Oncogene 2004; 23: 3338–3349.
Zeng Z, Samudio IJ, Zhang W, Estrov Z, Pelicano H, Harris D et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res 2006; 66: 3737–3746.
Kim KT, Baird K, Ahn JY, Meltzer P, Lilly M, Levis M et al. Pim-1 is up-regulated by constitutively activated FLT3 and plays a role in FLT3-mediated cell survival. Blood 2005; 105: 1759–1767.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther 2006; 5: 995–1006.
Guo J, Marcotte PA, McCall JO, Dai Y, Pease LJ, Michaelides MR et al. Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther 2006; 5: 1007–1013.
Shankar DB, Li J, Tapang P, Owen McCall J, Pease LJ, Dai Y et al. ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia. Blood 2007; 109: 3400–3408.
Druker BJ, Sawyers CL, Capdeville R, Ford JM, Baccarani M, Goldman JM . Chronic myelogenous leukemia. Hematology Am Soc Hematol Educ Program 2001, 87–112.
Yee KW, Schittenhelm M, O’Farrell AM, Town AR, McGreevey L, Bainbridge T et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood 2004; 104: 4202–4209.
Levis M, Pham R, Smith BD, Small D . In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood 2004; 104: 1145–1150.
Brown P, Levis M, McIntyre E, Griesemer M, Small D . Combinations of the FLT3 inhibitor CEP-701 and chemotherapy synergistically kill infant and childhood MLL-rearranged ALL cells in a sequence-dependent manner. Leukemia 2006; 20: 1368–1376.
Shen J, Tai YC, Zhou J, Stephen Wong CH, Cheang PT, Fred Wong WS et al. Synergistic antileukemia effect of genistein and chemotherapy in mouse xenograft model and potential mechanism through MAPK signaling. Exp Hematol 2007; 35: 75–83.
Abruzzo LV, Lee KY, Fuller A, Silverman A, Keating MJ, Medeiros LJ et al. Validation of oligonucleotide microarray data using microfluidic low-density arrays: a new statistical method to normalize real-time RT-PCR data. Biotechniques 2005; 38: 785–792.
Ionov Y, Le X, Tunquist BJ, Sweetenham J, Sachs T, Ryder J et al. Pim-1 protein kinase is nuclear in Burkitt's lymphoma: nuclear localization is necessary for its biologic effects. Anticancer Res 2003; 23: 167–178.
Gong J, Traganos F, Darzynkiewicz Z . Staurosporine blocks cell progression through G1 between the cyclin D and cyclin E restriction points. Cancer Res 1994; 54: 3136–3139.
Lakhani SA, Masud A, Kuida K, Porter Jr GA, Booth CJ, Mehal WZ et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 2006; 311: 847–851.
Musgrove EA . Cyclins: roles in mitogenic signaling and oncogenic transformation. Growth Factors 2006; 24: 13–19.
Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 1994; 265: 966–970.
Sagata N . What does Mos do in oocytes and somatic cells? Bioessays 1997; 19: 13–21.
Yang Y, Pham CD, Arlinghaus RB, Khillan JS, Singh B . Elevated level of cyclin D1 in mos-transformed cells. Int J Oncol 1998; 12: 1199–1202.
Li CC, Chen E, O’Connell CD, Longo DL . Detection of c-mos proto-oncogene expression in human cells. Oncogene 1993; 8: 1685–1691.
Parkar MH, Seid JM, Stringer BM, Ingemansson S, Woodhouse N, Goyns MH . Abnormal expression of the MOS proto-oncogene in human thyroid medullary carcinoma. Cancer Lett 1988; 43: 185–189.
Gorgoulis VG, Zacharatos P, Mariatos G, Liloglou T, Kokotas S, Kastrinakis N et al. Deregulated expression of c-mos in non-small cell lung carcinomas: relationship with p53 status, genomic instability, and tumor kinetics. Cancer Res 2001; 61: 538–549.
Lee Jr JT, McCubrey JA . The Raf/MEK/ERK signal transduction cascade as a target for chemotherapeutic intervention in leukemia. Leukemia 2002; 16: 486–507.
Grzanka A, Zuryn A, Styczynski J, Grzanka AA, Wisniewska H . The effect of doxorubicin on the expression of cyclin A in K-562 leukemia cell line. Neoplasma 2005; 52: 489–493.
Acknowledgements
We specially thank Professor Evelyn SC Koay for scientific discussion and Ms Khng Jiaying for administrative help, as well as Dr Richie Soong and Ms Baidah Binte Ahmad for excellent assistance in low-density array analysis. This work was supported by Singapore Cancer Syndicate Grant—TN0031, AN0038 and Terry Fox Run Cancer Research Grant 2004 (C-S Chen).
Author information
Authors and Affiliations
Corresponding author
Additional information
Declaration of commercial interest: Three of the authors (K.B.G., D.H.A. and S.K.D.) are employees of Abbott Laboratories, whose potential product was studied in the present work.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary information
Rights and permissions
About this article
Cite this article
Zhou, J., Pan, M., Xie, Z. et al. Synergistic antileukemic effects between ABT-869 and chemotherapy involve downregulation of cell cycle-regulated genes and c-Mos-mediated MAPK pathway. Leukemia 22, 138–146 (2008). https://doi.org/10.1038/sj.leu.2404960
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404960
Keywords
This article is cited by
-
Linifanib (ABT-869) Potentiates the Efficacy of Chemotherapeutic Agents through the Suppression of Receptor Tyrosine Kinase-Mediated AKT/mTOR Signaling Pathways in Gastric Cancer
Scientific Reports (2016)
-
Autophagy inhibition sensitizes hepatocellular carcinoma to the multikinase inhibitor linifanib
Scientific Reports (2014)
-
ABT-869, a promising multi-targeted tyrosine kinase inhibitor: from bench to bedside
Journal of Hematology & Oncology (2009)