Abstract
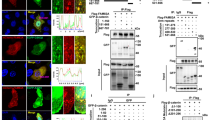
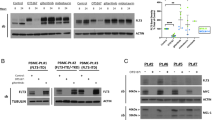
Deregulated accumulation of nuclear β-catenin enhances transcription of β-catenin target genes and promotes malignant transformation. Recently, acute myeloid leukemia (AML) cells with activating mutations of FMS-like tyrosine kinase-3 (FLT3) were reported to display elevated β-catenin-dependent nuclear signaling. Tyrosine phosphorylation of β-catenin has been shown to promote its nuclear localization. Here, we examined the causal relationship between FLT3 activity and β-catenin nuclear localization. Compared to cells with wild-type FLT3 (FLT3-WT), cells with the FLT3 internal tandem duplication (FLT3-ITD) and tyrosine kinase domain mutation (FLT3-TKD) had elevated levels of tyrosine-phosphorylated β-catenin. Although β-catenin was localized mainly in the cytoplasm in FLT3-WT cells, it was primarily nuclear in FLT3-ITD cells. Treatment with FLT3 kinase inhibitors or FLT3 silencing with RNAi decreased β-catenin tyrosine phosphorylation and nuclear localization. Conversely, treatment of FLT3-WT cells with FLT3 ligand increased tyrosine phosphorylation and nuclear accumulation of β-catenin. Endogenous β-catenin co-immunoprecipitated with endogenous activated FLT3, and recombinant activated FLT3 directly phosphorylated recombinant β-catenin. Finally, FLT3 inhibitor decreased tyrosine phosphorylation of β-catenin in leukemia cells obtained from FLT3-ITD-positive AML patients. These data demonstrate that FLT3 activation induces β-catenin tyrosine phosphorylation and nuclear localization, and thus suggest a mechanism for the association of FLT3 activation and β-catenin oncogeneic signaling in AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lyman SD, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 1993; 75: 1157–1167.
Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 1994; 368: 643–648.
Lavagna-Sevenier C, Marchetto S, Birnbaum D, Rosnet O . FLT3 signaling in hematopoietic cells involves CBL, SHC and an unknown P115 as prominent tyrosine-phosphorylated substrates. Leukemia 1998; 12: 301–310.
Zhang S, Broxmeyer HE . Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp-2, grb2, and PI3 kinase. Biochem Biophys Res Commun 2000; 277: 195–199.
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 1996; 10: 1911–1918.
Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia 1997; 11: 1605–1609.
Meshinchi S, Woods WG, Stirewalt DL, Sweetser DA, Buckley JD, Tjoa TK et al. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood 2001; 97: 89–94.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002; 99: 4326–4335.
Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001; 97: 2434–2439.
Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 2000; 19: 624–631.
Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 1998; 12: 1333–1337.
Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T . Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 2002; 21: 2555–2563.
Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 1999; 93: 3074–3080.
Nakano Y, Kiyoi H, Miyawaki S, Asou N, Ohno R, Saito H et al. Molecular evolution of acute myeloid leukaemia in relapse: unstable-and FLT3 genes compared with p53 gene. Br J Haematol 1999; 104: 659–664.
Ozawa M, Baribault H, Kemler R . The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8: 1711–1717.
Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM . The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 1992; 118: 681–691.
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA 1999; 96: 5522–5527.
Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature 1996; 382: 638–642.
Bienz M, Clevers H . Linking colorectal cancer to Wnt signaling. Cell 2000; 103: 311–320.
Barker N, Clevers H . Catenins, Wnt signaling and cancer. Bioessays 2000; 22: 961–965.
Easwaran V, Song V, Polakis P, Byers S . The ubiquitin-proteasome pathway and serine kinase activity modulate adenomatous polyposis coli protein-mediated regulation of beta-catenin-lymphocyte enhancer-binding factor signaling. J Biol Chem 1999; 274: 16641–16645.
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R . beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16: 3797–3804.
Tetsu O, McCormick F . Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398: 422–426.
Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG . Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem 2001; 276: 20436–20443.
Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ . Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the beta-catenin pathway. Mol Cell Biol 2001; 21: 5857–5868.
McWhirter JR, Neuteboom ST, Wancewicz EV, Monia BP, Downing JR, Murre C . Oncogenic homeodomain transcription factor E2A-Pbx1 activates a novel WNT gene in pre-B acute lymphoblastoid leukemia. Proc Natl Acad Sci USA 1999; 96: 11464–11469.
Chung EJ, Hwang SG, Nguyen P, Lee S, Kim JS, Kim JW et al. Regulation of leukemic cell adhesion, proliferation, and survival by beta-catenin. Blood 2002; 100: 982–990.
Derksen PW, Tjin E, Meijer HP, Klok MD, MacGillavry HD, van Oers MH et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc Natl Acad Sci USA 2004; 101: 6122–6127.
Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2004; 101: 3118–3123.
Simon M, Grandage VL, Linch DC, Khwaja A . Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene 2005; 24: 2410–2420.
Tickenbrock L, Schwable J, Wiedehage M, Steffen B, Sargin B, Choudhary C et al. Flt3 tandem duplication mutations cooperate with Wnt signaling in leukemic signal transduction. Blood 2005; 105: 3699–3706.
Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A et al. Geldanamycin abrogates ErbB2 association with proteasome-resistant beta-catenin in melanoma cells, increases beta-catenin-E-cadherin association, and decreases beta-catenin-sensitive transcription. Cancer Res 2001; 61: 1671–1677.
Ilan N, Tucker A, Madri JA . Vascular endothelial growth factor expression, beta-catenin tyrosine phosphorylation, and endothelial proliferative behavior: a pathway for transformation? Lab Invest 2003; 83: 1105–1115.
Zhao M, Kiyoi H, Yamamoto Y, Ito M, Towatari M, Omura S et al. In vivo treatment of mutant FLT3-transformed murine leukemia with a tyrosine kinase inhibitor. Leukemia 2000; 14: 374–378.
Grundler R, Thiede C, Miething C, Steudel C, Peschel C, Duyster J . Sensitivity toward tyrosine kinase inhibitors varies between different activating mutations of the FLT3 receptor. Blood 2003; 102: 646–651.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Harris TJ, Peifer M . Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol 2005; 15: 234–237.
Polakis P . Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851.
Aberle H, Schwartz H, Hoschuetzky H, Kemler R . Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J Biol Chem 1996; 271: 1520–1526.
Aono S, Nakagawa S, Reynolds AB, Takeichi M . p120(ctn) acts as an inhibitory regulator of cadherin function in colon carcinoma cells. J Cell Biol 1999; 145: 551–562.
Krieghoff E, Behrens J, Mayr B . Nucleo-cytoplasmic distribution of {beta}-catenin is regulated by retention. J Cell Sci 2006; 119: 1453–1463.
Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W . Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev 2004; 18: 2225–2230.
Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M . Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J Biol Chem 1999; 274: 36734–36740.
Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res 2006; 66: 11967–11974.
Chalandon Y, Schwaller J . Targeting mutated protein tyrosine kinases and their signaling pathways in hematologic malignancies. Haematologica 2005; 90: 949–968.
Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C . Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J 2007; 26: 1456–1466.
Sukhdeo K, Mani M, Zhang Y, Dutta J, Yasui H, Rooney MD et al. Targeting the beta-catenin/TCF transcriptional complex in the treatment of multiple myeloma. Proc Natl Acad Sci USA 2007; 104: 7516–7521.
Acknowledgements
We thank Dr Kazutaka Ozeki, Nagoya University School of Medicine, for his help in preparing MOLM-13 cells and stably transfected 32D cell lines. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. Support was also provided to TK by the Sumitomo Life Social Welfare Sciences Foundation. Statement of authorship: TK, LN, MJL, JBT designed research , TK, EJC, MJL, SL, AS performed research, TK wrote the paper, and HK, MJL, TN contributed vital new reagents.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kajiguchi, T., Chung, E., Lee, S. et al. FLT3 regulates β-catenin tyrosine phosphorylation, nuclear localization, and transcriptional activity in acute myeloid leukemia cells. Leukemia 21, 2476–2484 (2007). https://doi.org/10.1038/sj.leu.2404923
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404923
Keywords
This article is cited by
-
MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies
Journal of Hematology & Oncology (2021)
-
Targeting nuclear β-catenin as therapy for post-myeloproliferative neoplasm secondary AML
Leukemia (2019)
-
Pre-clinical efficacy of combined therapy with novel β-catenin antagonist BC2059 and histone deacetylase inhibitor against AML cells
Leukemia (2015)
-
γ-Catenin is overexpressed in acute myeloid leukemia and promotes the stabilization and nuclear localization of β-catenin
Leukemia (2013)
-
Neonatal tumours
Pediatric Surgery International (2013)