Abstract
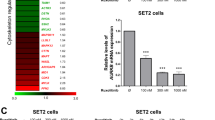
Anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALK+ ALCL) is characterized by constitutive activation of the Janus kinase (JAK)3/signal transducers and activators of transcription 3 (STAT3) signaling pathway. SHP1, a tyrosine phosphatase that negatively regulates JAK/STAT, is frequently absent in ALK+ ALCL owing to gene methylation. To test the hypothesis that loss of SHP1 contributes to JAK3/STAT3 activation in ALK+ ALCL cells, we induced SHP1 expression using 5-aza-2′-deoxycytidine (5-AZA), an inhibitor of DNA methyltransferase, in ALK+ ALCL cell lines, and correlated with changes in the JAK3/STAT3 pathway. 5-AZA gradually restored SHP1 expression in Karpas 299 and SU-DHL-1 cells over 5 days. The initially low level of SHP1 expression did not result in significant changes to the expression or tyrosine phosphorylation of JAK3 and STAT3. However, higher levels of SHP1 seen subsequently correlated with substantial decreases in JAK3 and pJAK3, followed by pSTAT3 (but not STAT3). Importantly, the decrease in JAK3 was abrogated by MG132, a proteasome inhibitor. 5-AZA induced no significant increase in apoptosis but it sensitized ALCL cells to doxorubicin-induced apoptosis. Our findings support the concept that loss of SHP1 contributes to the constitutive activation of JAK3/STAT3 in ALK+ ALCL cells. SHP1 appears to downregulate JAK3 by two mechanisms: tyrosine dephosphorylation and increased degradation via the proteasome pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Skinnider BF, Connors JM, Sutcliffe SB, Gascoyne RD . Anaplastic large cell lymphoma. A clinicopathologic analysis. Hematol Oncol 1999; 17: 137–148.
Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994; 263: 281–284.
Fujimoto J, Shiota M, Iwahara T, Seki N, Satoh H, Mori S et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996; 93: 4181–4186.
Bischof D, Pulford K, Mason DY, Morris SW . Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol 1997; 4: 2312–2325.
Bai RY, Dieter P, Peschel C, Morris SW, Duyster J . Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol Cell Biol 1998; 18: 6951–6961.
Duyster J, Bai RY, Morris SW . Translocations involving anaplastic lymphoma kinase (ALK). Oncogene 2001; 20: 5623–5637.
Slupianek A, Nieborowska-Skorska M, Hoser G, Morrione A, Majewski M, Xue L et al. Role of phosphatidylinositol 3-kinase-Akt pathway in nucleophosmin/anaplastic lymphoma kinase-mediated lymphomagenesis. Cancer Res 2001; 61: 2194–2199.
Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol 2002; 168: 466–474.
Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M et al. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002; 21: 1038–1047.
Amin HM, McDonnell TJ, Ma Y, Lin Q, Fujio Y, Kunisada K et al. Selective inhibition of STAT3 induces apoptosis and G (1) cell cycle arrest in ALK-positive anaplastic large cell lymphoma. Oncogene 2004; 23: 5426–5434.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse JF, Capiod JC, Delobel J et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood 1996; 87: 1692–1697.
Chai SK, Nichols GL, Rothman P . Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol 1997; 159: 4720–4728.
Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ 1997; 8: 1267–1276.
Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth in vitro. J Clin Invest 1998; 102: 1385–1392.
Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, Leventaki V et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK− anaplastic large cell lymphoma. Clin Cancer Res 2003; 9: 3692–3699.
Chiarle R, Simmons WJ, Cai H, Dhall G, Zamo A, Raz R et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med 2005; 11: 595–596.
Cussac D, Greenland C, Roche S, Bai RY, Duyster J, Morris SW et al. Nucleophosmin-anaplastic lymphoma kinase of anaplastic large-cell lymphoma recruits, activates, and uses pp60c-src to mediate its mitogenicity. Blood 2004; 103: 1464–1471.
Amin HM, Medeiros LJ, Ma Y, Feretzaki M, Das P, Leventaki V et al. Inhibition of JAK3 induces apoptosis and decreases anaplastic lymphoma kinase activity in anaplastic large cell lymphoma. Oncogene 2003; 22: 5399–5407.
Zhang Q, Raghunath PN, Vonderheid E, Odum N, Wasik MA . Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter. Am J Pathol 2000; 157: 1137–1146.
Matthews RJ, Bowne DB, Flores E, Thomas ML . Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol 1992; 12: 2396–2405.
Plutzky J, Neel BG, Rosenberg RD . Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci USA 1992; 89: 1123–1127.
Neel BG, Tonks NK . Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol 1997; 9: 193–204.
Tonks NK, Neel BG . From form to function: signaling by protein tyrosine phosphatases. Cell 1996; 87: 365–368.
Zhang J, Somani AK, Siminovitch KA . Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signaling. Semin Immunol 2000; 12: 361–378.
Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF . Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 1995; 80: 729–738.
Bittorf T, Seiler J, Zhang Z, Jaster R, Brock J . SHP1 protein tyrosine phosphatase negatively modulates erythroid differentiation and suppression of apoptosis in J2E erythroleukemic cells. Biol Chem 1999; 380: 1201–1209.
Townley R, Shen SH, Banville D, Ramachandran C . Inhibition of the activity of protein tyrosine phosphate 1C by its SH2 domains. Biochemistry 1993; 32: 13414–13418.
Pei D, Wang J, Walsh CT . Differential functions of the two Src homology 2 domains in protein tyrosine phosphatase SH-PTP1. Proc Natl Acad Sci USA 1996; 93: 1141–1145.
Wu C, Sun M, Liu L, Zhou GW . The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene 2003; 306: 1–12.
Khoury JD, Rassidakis GZ, Medeiros LJ, Amin HM, Lai R . Methylation of SHP1 gene and loss of SHP1 protein expression are frequent in systemic anaplastic large cell lymphoma. Blood 2004; 104: 1580–1581.
Epstein AL, Kaplan HS . Biology of the human malignant lymphomas. I. Establishment in continuous cell culture and heterotransplantation of diffuse histiocytic lymphomas. Cancer 1974; 34: 1851–1872.
Morgan R, Smith SD, Hecht BK, Christy V, Mellentin JD, Warnke R et al. Lack of involvement of the c-fms and N-myc genes by chromosomal translocation t(2;5)(p23;q35) common to malignancies with features of so-called malignant histiocytosis. Blood 1989; 73: 2155–2164.
Andrews III DF, Nemunaitis J, Tompkins C, Singer JW . Effect of 5-azacytidine on gene expression in marrow stromal cells. Mol Cell Biol 1989; 9: 2748–2751.
Momparler RL, Cote S, Eliopoulos N . Pharmacological approach for optimization of the dose schedule of 5-Aza-2′-deoxycytidine (Decitabine) for the therapy of leukemia. Leukemia 1997; 11 (Suppl 1): S1–S6.
Ma XZ, Jin T, Sakac D, Fahim S, Zhang X, Katsman Y et al. Abnormal splicing of SHP-1 protein tyrosine phosphatase in human T cells. Implications for lymphomagenesis. Exp Hematol 2003; 31: 131–142.
Wu C, Guan Q, Wang Y, Zhao ZJ, Zhou GW . SHP-1 suppresses cancer cell growth by promoting degradation of JAK kinases. J Cell Biochem 2003; 90: 1026–1037.
Zhang Q, Wang HY, Marzec M, Raghunath PN, Nagasawa T, Wasik MA . STAT3 and DNA methyltransferase 1-mediated epigenetic silencing of SHP1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci USA 2005; 10: 6948–6953.
Oka T, Yoshino T, Hayashi K, Ohara N, Nakanishi T, Yamaai Y et al. Reduction of hematopoietic cell-specific tyrosine phosphatase SHP-1 gene expression in natural killer cell lymphoma and various types of lymphomas/leukemias: combination analysis with cDNA expression array and tissue microarray. Am J Pathol 2001; 159: 1495–1505.
Oka T, Ouchida M, Koyama M, Ogama Y, Takada S, Nakatani Y et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res 2002; 62: 6390–6394.
Altieri DC . The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol Med 2001; 7: 542–547.
Mahboubi K, Li F, Plescia J, Kirkiles-Smith NC, Mesri M, Du Y et al. Interleukin-11 up-regulates survivin expression in endothelial cells through a signal transducer and activator of transcription-3 pathway. Lab Invest 2001; 81: 327–334.
Aoki Y, Feldman GM, Tosato G . Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood 2003; 101: 1535–1542.
Lavelle D, DeSimone J, Hankewych M, Kousnetzova T, Chen YH . Decitabine induces cell cycle arrest at the G1 phase via p21 (WAF1) and the G2/M phase via the p38 MAP kinase pathway. Leuk Res 2003; 27: 999–1007.
Honorat J, Ragab A, Lamant L, Delsol G, Ragab-Thomas J . SHP1 tyrosine phosphatase negatively regulates NPM-ALK tyrosine kinase signaling. Blood 2006; 107: 4130–4138.
Acknowledgements
This study is supported by research grants from the Alberta Cancer Board and the University of Alberta Hospital Research Foundation awarded to RL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Y., Amin, H., Frantz, C. et al. Restoration of shp1 expression by 5-AZA-2′-deoxycytidine is associated with downregulation of JAK3/STAT3 signaling in ALK-positive anaplastic large cell lymphoma. Leukemia 20, 1602–1609 (2006). https://doi.org/10.1038/sj.leu.2404323
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404323
Keywords
This article is cited by
-
STAT3 activation in large granular lymphocyte leukemia is associated with cytokine signaling and DNA hypermethylation
Leukemia (2021)
-
Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma
Clinical Epigenetics (2020)
-
Prognostic and therapeutic significance of phosphorylated STAT3 and protein tyrosine phosphatase-6 in peripheral-T cell lymphoma
Blood Cancer Journal (2018)
-
Genome-wide analyses identify KLF4 as an important negative regulator in T-cell acute lymphoblastic leukemia through directly inhibiting T-cell associated genes
Molecular Cancer (2015)
-
Tumor antigen ROR1 targeted drug delivery mediated selective leukemic but not normal B-cell cytotoxicity in chronic lymphocytic leukemia
Leukemia (2015)