Abstract
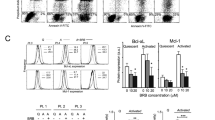
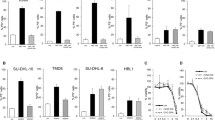
The effects of the hyperforin (HF), a natural phloroglucinol purified from Hypericum perforatum, were investigated ex vivo on leukemic cells from patients with B-cell chronic lymphocytic leukemia (B-CLL). HF was found to promote apoptosis of B-CLL cells, as shown by time- and dose-dependent stimulation of phosphatidylserine externalization and DNA fragmentation, by disruption of the mitochondrial transmembrane potential, caspase-3 activation and cleavage of the caspase substrate PARP-1. Moreover, HF-induced downregulation of Bcl-2 and Mcl-1, two antiapoptotic proteins that control mitochondrial permeability. HF also downregulated two proteins which are overexpressed by B-CLL patients' cells, the cell cycle inhibitor p27kip1 through caspase-dependent cleavage into a p23 form, and the nitric oxid (NO) synthase of type 2 (inducible NO synthase). This latter was accompanied by reduction in the production of NO known to be antiapoptotic in B-CLL cells. Preventing effects of the general caspase inhibitor z-VAD-fmk indicated that HF-promoted apoptosis of B-CLL cells was mostly caspase dependent. Furthermore, normal B lymphocytes purified from healthy donors appeared less sensitive to HF-induced apoptosis than B-CLL cells. These results indicate that HF may be of interest in the development of new therapies for B-CLL based on the induction of apoptosis and combination with cell cycle-dependent antitumor drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barnes J, Anderson LA, Phillipson JD . St John's wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol 2001; 53: 583–600.
Gobbi M, Mennini T . Is St John's wort a ‘Prozac- like’ herbal antidepressant? Trends Pharmacol Sci 2001; 22: 557–559.
Muller WE . Current St John's wort research from mode of action to clinical efficacy. Pharmacol Res 2003; 47: 101–109.
Singer A, Wonnemann M, Muller WE . Hyperforin, a major antidepressant constituent of St. John's Wort, inhibits serotonin uptake by elevating free intracellular Na+. J Pharmacol Exp Ther 1999; 290: 1363–1368.
Treiber K, Singer A, Henke B, Muller WE . Hyperforin activates nonselective cation channels (NSCCs). Br J Pharmacol 2005; 145: 75–83.
Schempp CM, Pelz K, Wittmer A, Schopf E, Simon JC . Antibacterial activity of hyperforin from St John's wort, against multiresistant Staphylococcus aureus and gram-positive bacteria. Lancet 1999; 353: 2129.
Heilmann J, Winkelmann K, Sticher O . Studies on the antioxidative activity of phloroglucinol derivatives isolated from hypericum species. Planta Med 2003; 69: 202–206.
Zou L, Harkey MR, Henderson GL . Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci 2002; 71: 1579–1589.
Komoroski BJ, Zhang S, Cai H, Hutzler JM, Frye R, Tracy TS et al. Induction and inhibition of cytochromes p450 by the St. John's wort constituent hyperforin in human hepatocyte cultures. Drug Metab Dispos 2004; 32: 512–518.
Albert D, Zundorf I, Dingermann T, Muller WE, Steinhilber D, Werz O . Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochem Pharmacol 2002; 64: 1767–1775.
Schempp CM, Winghofer B, Ludtke R, Simon-Haarhaus B, Schopf E, Simon JC . Topical application of St John's wort (Hypericum perforatum L.) and of its metabolite hyperforin inhibits the allostimulatory capacity of epidermal cells. Br J Dermatol 2000; 142: 979–984.
Hostanska K, Reichling J, Bommer S, Weber M, Saller R . Aqueous ethanolic extract of St. John's wort (Hypericum perforatum L.) induces growth inhibition and apoptosis in human malignant cells in vitro. Pharmazie 2002; 57: 323–331.
Schempp CM, Kirkin V, Simon-Haarhaus B, Kersten A, Kiss J, Termeer CC et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John's wort that acts by induction of apoptosis. Oncogene 2002; 21: 1242–1250.
Hostanska K, Reichling J, Bommer S, Weber M, Saller R . Hyperforin a constituent of St John's wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm 2003; 56: 121–132.
Bannerji R, Byrd JC . Update on the biology of chronic lymphocytic leukemia. Curr Opin Oncol 2000; 12: 22–29.
Kolb JP, Kern C, Quiney C, Roman V, Billard C . Re-establishment of a normal apoptotic process as a therapeutic approach in B-CLL. Curr Drug Targets Cardivasc Haematol Disord 2003; 3: 261–286.
Zhao H, Dugas N, Mathiot C, Dugas B, Sigaux F, Kolb JP . B-cell chronic lymphocytic leukemia cells express a functional inducible nitric oxide synthase displaying anti-apoptotic activity. Blood 1998; 92: 1031–1043.
Kern C, Cornuel J, Billard C, Tang R, Rouillard D, Steunou V et al. Involvement of BAFF and APRIL in the resistance to apoptosis of B-CLL through an autocrine pathway. Blood 2004; 103: 679–688.
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH . Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84: 1415–1420.
Billard C, Izard JC, Roman V, Kern C, Mathiot C, Mentz F et al. Comparative antiproliferative and apoptotic effects of resveratrol, ɛ-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal lymphocytes. Leuk Lymphoma 2002; 43: 1991–2002.
Billard C, Kern C, Tang R, Ajchenbaum-Cymbalista F, Kolb JP . Flavopiridol downregulates the expression of both the inducible NO synthase and p27kip1 in malignant cells from B-cell chronic lymphocytic leukemia. Leukemia 2003; 17: 2345–2443.
Roman V, Billard C, Kern C, Ferry-Dumazet H, Izard JC, Mohammad R et al. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukemia. Br J Haematol 2002; 117: 1–10.
Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with in vitro and in vivo chemoresponses. Blood 1998; 91: 3379–3389.
Levesque MC, Misukonis MA, O'Loughlin CW, Chen Y, Beasley BE, Wilson DL et al. IL-4 and interferon gamma regulate expression of inducible nitric oxide synthase in chronic lymphocytic leukemia cells. Leukemia 2003; 17: 442–450.
Vrhorac R, Delmer A, Tang R, Marie JP, Zittoun R, Ajchenbaum-Cymbalista F . Prognostic significance of the cell cycle inhibitor p27Kip1 in chronic lymphocytic leukemia. Blood 1998; 91: 4694–4700.
Frost V, Sinclair AJ . p27KIP1 is down-regulated by two different mechanisms in human lymphoid cells undergoing apoptosis. Oncogene 2000; 19: 3115–3120.
Butterweck V, Nahrstedt A, Evans J, Hufeisen S, Rauser L, Savage J et al. In vitro receptor screening of pure constituents of St. John's wort reveals novel interactions with a number of GPCRs. Psychopharmacology (Berlin) 2002; 162: 193–202.
Kolb JP, Roman V, Mentz F, Zhao H, Rouillard D, Dugas N et al. Contribution of nitric oxide to the apoptotic process in human B cell chronic lymphocytic leukemia. Leuk Lymphoma 2001; 40: 243–257.
Quiney C, Dauzonne D, Kern C, Fourneron JD, Izard JC, Mohammad RM et al. Flavones and polyphenols inhibit the NO pathway during apoptosis of leukemia B-cells. Leuk Res 2004; 28: 851–861.
Dimmeler S, Haendeler J, Nehls M, Zeiher AM . Suppression of apoptosis by nitric oxide via inhibition of interleukin-1beta converting enzymes (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J Exp Med 1997; 185: 601–607.
Lin TS, Porcu P . Flavopiridol: where do we stand in chronic lymphocytic leukemia? Leukemia 2004; 18: 243–246.
Gartner M, Muller T, Simon JC, Giannis A, Sleeman JP . Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent. Chembiochem 2005; 6: 171–177.
Dona M, Dell'Aica I, Pezzato E, Sartor L, Calabrese F, Della Barbera M et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res 2004; 64: 6225–6232.
Acknowledgements
We thank Mrs S Pasco-Dubrulle for her skillful assistance and the clinicians of the Department of Hematology at the Hôtel-Dieu hospital. This work was supported by INSERM, Canceropôle Ile-de-France and by a grant from ARC (no. 3322).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quiney, C., Billard, C., Faussat, A. et al. Pro-apoptotic properties of hyperforin in leukemic cells from patients with B-cell chronic lymphocytic leukemia. Leukemia 20, 491–497 (2006). https://doi.org/10.1038/sj.leu.2404098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404098
Keywords
This article is cited by
-
The acylphloroglucinols hyperforin and myrtucommulone A cause mitochondrial dysfunctions in leukemic cells by direct interference with mitochondria
Apoptosis (2015)
-
Design of novel BH3 mimetics for the treatment of chronic lymphocytic leukemia
Leukemia (2012)
-
Noxa upregulation is associated with apoptosis of chronic lymphocytic leukemia cells induced by hyperforin but not flavopiridol
Leukemia (2009)
-
Hyperforin inhibits MMP-9 secretion by B-CLL cells and microtubule formation by endothelial cells
Leukemia (2006)
-
Hyperforin, a new lead compound against the progression of cancer and leukemia?
Leukemia (2006)