Abstract
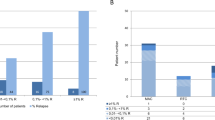
Both conventional chimerism analysis (CCA) and lineage-specific chimerism analysis (LCA) have potential pitfalls as diagnostic means for the detection of minimal residual disease after allogeneic hematopoietic cell transplantation (aHCT). Therefore, the present study examines the results of both methods in order to determine how predictive consecutive evaluations were, with respect to the risk that the patient would relapse during post-transplant follow-up and with respect to responsiveness to immunomodulatory treatment. A total of 168 individuals with acute myeloid leukemia (AML) (n=137) and myelo dysplastic syndrome (n=31) were investigated with CCA and LCA at mean intervals of 24 days (range: 11–116). The median follow-up after myeloablative aHCT was 22 months (range: 4–49). Of 168 patients, 65 experienced a clinical relapse after aHCT. CCA and LCA were comparatively sensitive and specific for relapse at the intervals of chimerism testing employed in this study. Of 32 patients, 10 who were offered donor lymphocyte infusions (DLI) treatment for increasing (n=29) or stable (n=3) mixed chimerism (MC) achieved at least transitory CC. The observation that all patients with increasing MC relapsed despite DLI treatment (54%) or withdrawal of immune suppression (24%) indicates that novel strategies to deal with rapidly evolving relapse in AML patients, such as shortening of chimerism monitoring intervals, need to be evaluated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood 1989; 73: 1720–1728.
van Rhee F, Lin F, Cullis JO, Spencer A, Cross NC, Chase A et al. Relapse of chronic myeloid leukemia after allogeneic bone marrow transplant: the case for giving donor leukocyte transfusions before the onset of hematologic relapse. Blood 1994; 83: 3377–3383.
Dazzi F, Szydlo RM, Goldman JM . Donor lymphocyte infusions for relapse of chronic myeloid leukemia after allogeneic stem cell transplant: where we now stand. Exp Hematol 1999; 27: 1477–1486.
Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 1999; 13: 2079–2086.
Salama M, Nevill T, Marcellus D, Parker P, Johnson M, Kirk A et al. Donor leukocyte infusions for multiple myeloma. Bone Marrow Transplant 2000; 26: 1179–1184.
Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol 2000; 18: 3031–3037.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia (see comments). Blood 1995; 86: 2041–2050.
van Besien KW, Lima M, Giralt SA, Moore Jr DF, Khouri IF, Rondon G et al. Management of lymphoma recurrence after allogeneic transplantation: the relevance of graft-versus-lymphoma effect. Bone Marrow Transplantation 1997; 19: 977–982.
Mackinnon S, Barnett L, Bourhis JH, Black P, Heller G, O'Reilley RJ . Myeloid and lymphoid chimaerism after T-cell depleted bone marrow transplantation: evaluation of conditioning regimens using the polymerase chain reaction to amplify human minisatellite regions of genomic DNA. Blood 1992; 80: 3235–3241.
van Leeuwen JEM, van Tol MJD, Joosten AM, Wijnen JT, Verweij PJ, Khan PM et al. Persistence of host-type hematopoiesis after allogeneic bone marrow transplantation for leukemia is significantly related to the recipient's age and/or the conditioning regimen, but is not associated with an increased risk of relapse. Blood 1994; 83: 3059–3067.
Mattsson J, Uzunel M, Tammik L, Aschan J, Ringdén O . Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia 2001; 15: 1976–1985.
Lion T, Daxberger H, Dubovsky J, Filipcik P, Fritsch G, Printz D et al. Analysis of chimersim within specific leukocyte subsets for detection of residual or recurrent leukemia in pediatric patients after allogeneic stem cell transplantation. Leukemia 2001; 15: 307–310.
Thiede CH, Lutterbeck K, Oelschlagel U, Kiehl M, Steudel CH, Platzbecker U et al. Detection of relapse by sequential monitoring of chimerism circulating CD34+ cells. Ann Hematol 2002; 81 (Suppl 2): 27–28.
Finke J, Bertz H, Schmoor C, Veelken H, Behringer D, Wäsch R et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br J Haematol 2000; 111: 303–313.
Wäsch R, Reisser S, Hahn J, Bertz H, Engelhardt M, Kunzmann R et al. Rapid achievement of complete donor chimerism and low regimen-related toxicity after reduced conditioning with fludarabine, carmustine, melphalan and allogeneic transplantation. Bone Marrow Transplant 2000; 26: 243–250.
Greenberg P, Anderson J, de Witte T, Estey E, Fenaux P, Gupta P et al. Problematic WHO reclassification of myelodysplastic syndromes. Members of the International MDS Study Group. J Clin Oncol 2000; 18: 3447–3452.
Polymeropoulos MH, Rath DS, Xiao H, Merril CR . Tetranucleotide repeat polymorphism at the human beta-actin related pseudogene H-beta-Ac-psi-2 (ACTBP2). Nucleic Acids Res 1992; 20: 1432.
Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R . Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics 1992; 12: 241–253.
Budowle B, Chakraborty R, Giusti AM, Eisenberg AJ, Allen RC . Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 1991; 48: 137–144.
Gerken SC, Matsumani N, Lawrence E, Moore M, Ballard L, Melis R et al. Genetic and physical mapping of a simple repeat containing sequence tagged sites from the human genome. Genome Data Base Accession ID: GDB 198597, 1993.
Mastana S, Lee D, Singh PP, Singh M . Molecular genetic variation in the East Midlands, England: analysis of VNTR, STR and Alu insertion/deletion polymorphisms. Ann Hum Biol 2003; 30: 538–550.
Marubini E, Valsecchi MG . Analysing Survival Data from Clinical Trials and Observational Studies. John Wiley Press: New York, 1995.
Pepe MS . The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford University Press: New York, NY, 2003.
Kolb HJ . Donor leukocyte transfusions for treatment of leukemic relapse after bone marrow transplantation. EBMT Immunology and Chronic Leukemia Working Parties. Vox Sang 1998; 74: 321–329.
Pratt W, Ruddon R, Ensminger W, Maybaum J . Determinants of Drug Responsiveness. Oxford University Press: New York, NY, 1994.
Elmaagacli AH, Beelen DW, Kroll M, Trzensky S, Stein C, Schaefer UW . Detection of CBFbeta/MYH11 fusion transcripts in patients with inv (16) acute myeloid leukemia after allogeneic bone marrow or peripheral blood progenitor cell transplantation. Bone Marrow Transplant 1998; 21: 159–166.
Krauter J, Gorlich K, Ottmann O, Lubbert M, Dohner H, Heit W et al. Prognostic value of minimal residual disease quantification by real-time reverse transcriptase polymerase chain reaction in patients with core binding factor leukemias. J Clin Oncol 2003; 21: 4413–4422.
Campana D, Pui CH . Detection of minimal residual disease in acute leukemia: methodologic advances and clinical significance. Blood 1995; 85: 1416–1434.
Schattenberg A, De Witte T, Salden M, Vet J, Van Dijk B, Smeets D et al. Mixed hematopoietic chimerism after allogeneic transplantation with lymphocyte-depleted bone marrow is not associated with a higher incidence of relapse. Blood 1989; 73: 1367–1372.
van Leeuwen JE, van Tol MJ, Joosten AM, Wijnen JT, Khan PM, Vossen JM . Mixed T-lymphoid chimerism after allogeneic bone marrow transplantation for hematologic malignancies of children is not correlated with relapse. Blood 1993; 82: 1921–1928.
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 1998; 21: 487–495.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy. J Clin Oncol 2004; 22: 1696–1706.
Zetterquist H, Mattsson J, Uzunel M, Näsman-Björk I, Svenberg P, Tammik L et al. Mixed chimerism in the B cell lineage is a rapid and sensitive indicator of minimal residual disease in bone marrow transplant recipients with pre-B-cell acute lymphoblastic leukemia. Bone Marrow Transplant 2000; 25: 843–851.
Dubovsky J, Daxberger H, Fritsch G, Printz D, Peters C, Matthes S et al. Kinetics of chimerism during the early post-transplant period in pediatric patients with malignant and non-malignant hematologic disorders: implications for timely detection of engraftment, graft failure and rejection. Leukemia 1999; 13: 2059–2060.
Lamb Jr LS, Robbins NF, Abhyankar S, Joyce M, Stetler-Stevenson M, Henslee-Downey PJ et al. Flow cytometric cell sorting combined with molecular chimerism analysis to detect minimal recurrent leukemia: good news and bad news. Bone Marrow Transplant 1997; 19: 1157–1161.
Estrov Z, Ouspenskaia MV, Felix EA, McClain KL, Lee MS, Harris D et al. Persistence of self-renewing leukemia cell progenitors during remission in children with B-precursor acute lymphoblastic leukemia. Leukemia 1994; 8: 46–52.
Lauria F, Raspadori D, Ventura MA, Rondelli D, Testoni N, Tosi P et al. The presence of lymphoid-associated antigens in adult acute myeloid leukemia is devoid of prognostic relevance. Stem Cells 1995; 13: 428–434.
Goulden NJ, Knechtli CJ, Garland RJ, Langlands K, Hancock JP, Potter MN et al. Minimal residual disease analysis for the prediction of relapse in children with standard-risk acute lymphoblastic leukaemia. Br J Haematol 1998; 100: 235–244.
Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B . Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature 1987; 328: 429–432.
Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF . Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood 1997; 90: 4206–4211.
Acknowledgements
We are grateful to Dr Marie Follo for carefully proof-reading this paper. We appreciate the support of Mrs E Samek for cell sorting and Mrs S Krüger for PCR analysis and the transplant coordinator Mrs Lenartz, the data coordinator Mrs. Matt and the entire transplantation staff of the Department of Hematology and Oncology of the Freiburg University Medical Center for their excellent support and patient care. This project was supported by the Deutsche José Carreras Leukämie Stiftung e.V. (DJCLS Grant No. R00/11 to JF). RZ is supported by a fellowship grant from the Dr Mildred Scheel Stiftung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeiser, R., Spyridonidis, A., Wäsch, R. et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia 19, 814–821 (2005). https://doi.org/10.1038/sj.leu.2403719
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403719
Keywords
This article is cited by
-
Chimerism analysis for clinicians: a review of the literature and worldwide practices
Bone Marrow Transplantation (2022)
-
Monitoring minimal residual/relapsing disease after allogeneic haematopoietic stem cell transplantation in adult patients with acute lymphoblastic leukaemia
Bone Marrow Transplantation (2020)
-
Chimerism analysis in peripheral blood using indel quantitative real-time PCR is a useful tool to predict post-transplant relapse in acute leukemia
Bone Marrow Transplantation (2015)
-
A fast and simple approach for the simultaneous detection of hematopoietic chimerism, NPM1, and FLT3-ITD mutations after allogeneic stem cell transplantation
Annals of Hematology (2014)
-
Relapse assessment following allogeneic SCT in patients with MDS and AML
Annals of Hematology (2014)