Abstract
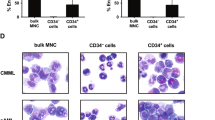
G-CSF primed CD34 cells cultured for 2–3 weeks in IL-2 and stem cell factor generate CD56high cells with phenotypic and morphologic features of NK cells, and a novel adherent CD56low CD16− population expressing myeloid markers (CD33 and HLA-DR). We hypothesized that similar cells might also occur in peripheral blood. In 13/13 normal individuals, we found a circulating population of CD56low, CD33+, FcγRI+, FcγRII+, HLA-DR+, CD11bhigh, CD14+ monocytes closely resembling the cultured CD56lowCD33+ cells. They may represent a normal counterpart of the CD56+ CD33+ hybrid myeloid/natural killer cell leukemia. Their mean frequency was 1.3±1% (standard deviation), range 0.16–3.5%, of total mononuclear cells. CD56lowCD33+ cells, primed with cytomegalovirus antigen, induced autologous T-lymphocyte proliferation comparably to CD56−, CD14+ peripheral blood monocytes (PBM). Conversely, CD56low cells induced greater T-cell proliferation than CD56− PBM when lymphocyte responders were HLA mismatched. Unstimulated CD56lowCD33+ cells showed a low antiproliferative effect on K562, which was increased upon LPS stimulation. The pattern of cytokine production by CD56lowCD33+ cells and PBM largely overlapped; however, they produced detectable levels of IL-6 and IL-1β. These results define a minor monocyte population with distinct phenotypic and functional features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Edelman GM . Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol 1986; 2: 81–116.
Edelman GM . Morphoregulatory molecules. Biochemistry 1988; 27: 3533–3543.
Comeau MR, Van der Vuurst de Vries AR, Maliszewski CR, Galimbert L . CD123 bright plasmacytoid predendritic cells: progenitors undergoing cell fate coversion? J Immunol 2002; 169: 75–83.
Miller JS, Verfaillie C, McGlave P . The generation of human natural killer cells from CD34+/DR− primitive progenitors in long-term bone marrow culture. Blood 1992; 80: 2182–2187.
Mrozek E, Anderson P, Caligiuri MA . Role of Interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitors cells. Blood 1996; 87: 2632–2640.
Sconocchia G, Fujiwara H, Rezvani K, Keyvanfar K, El Ouriaghli F, Grube M et al. G-CSF mobilized CD34+ cells cultured in interleukin-2 and stem cell factor generate a phenotypically novel monocyte. J Leuk Biol 2004; in press.
McKenzie D . Alloantigen presentation by B cells. J Immunol 1988; 141: 2907–2911.
Renauld JC, Vink A, VAN Snick J . Accessory signals in murine cytolytic T cell responses: dual requirement for IL-1 and IL-6. J Immunol 1989; 143: 1894–1898.
Kembala M, Uracz W, Ruggiero I, Mytar B, Pryjma J . Isolation and functional characteristics of FcR+ and FcR− human monocyte subsets. J Immunol 1984; 133: 1293–1299.
Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF . Subpopulation of human natural killer cells defined by expression of the LEU-7 (HNK-1) and LEU-11 (NK-15) antigens. J Immunol 1983; 131: 1789–1796.
Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH . The relationship of CD16 (LEU-11) and LEU-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 1986; 136: 4480–4486.
Phillips JH, Lanier LL . Dissection of the lymphokine-activated killer phenomenon. J Exp Med 1986; 164: 814–825.
Ikushima S, Yoshihara T, Matsumura T, Misawa S, Morioka Y, Hibi S et al. Expression of CD56/NCAM on hematopoietic malignant cells. A useful marker for acute monocytic and megakaryocytic leukemias. Int J Hematol 1991; 54: 395–403.
Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 2001; 15: 1161–1164.
Petrella T, Comeau MR, Maynadie' M, Couillault G, De Muret A, Maliszewski CR et al. Agranular CD4+CD56+ hematodermic neoplasm (blastic NK-cell lymphoma) originates from a population of CD56+ precursor related to plasmacytoid monocytes. Am J Surg Pathol 2002; 26: 852–862.
Nitta T, Yagita H, Sato K, Okumura K . Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer–target cell interaction. J Exp Med 1989; 170: 1757–1761.
Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH . Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N/CAM/CD56). J Immunol 1991; 12: 4421–4426.
Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW et al. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood 1999; 94: 3864–3871.
Suzuki R, Yamamoto K, Seto M, Kagami Y, Ogura M, Yatabe Y et al. CD7+ and CD56+ myeloid/natural killer cell precursor acute leukemia: a distinct hematolymphoid entity. Blood 1997; 90: 2417–2428.
Feuillard J, Jacob MC, Valensi F, Maynadie' M, Gressin R, Chaperot L et al. Clinical and biologic features of CD4+CD56+ malignancies. Blood 2003; 99: 1556–1563.
Penven K, Macro M, Salaun V, Comoz F, Reman O, Leroy D et al. Skin manifestation in CD4+CD56+ malignancies. Eur J Dermatol 2003; 13: 161–165.
Ziegler-Heitbrock HWL, Passlick B, Flieger D . The monoclonal antimonocyte antibody My4 stains B lymphocytes and two distinct monocytes subsets in human peripheral blood. Hybridoma 1988; 7: 521–527.
Passlick B, Flieger D, Ziegler-Heitbrock HWL . Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989; 74: 2527–2534.
Ziegler-Heitbrock HWL, Fingerle G, Strobel M, Schraut W, Stelter F, Schuett C et al. The novel subset of CD14+CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol 1993; 23: 2053–2058.
Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N . CD14lowCD16high: a cytokine-producing monocytes subset expands during human immunodeficiency virus infection. Eur J Immunol 1995; 25: 3418–3424.
Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS . Unique monocyte subset in patients with AIDS dementia. Lancet 1997; 349: 692–695.
Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002; 168: 3536–3542.
Ziegler-Heitbrock HWL . Heterogeneity of human blood monocytes: the CD14+CD16+ subpopulation. Immunol Today 1999; 17: 424–428.
Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad HD, Ernst M . Identification of a novel dendritic cell-like subset of CD64+/CD16+ blood monocytes. Eur J Immunol 2001; 31: 48–56.
Keller R . Macrophage-mediated natural cytotoxicity against various target cells in vitro. II. Macrophages from rats of different ages. Br J Haematol 1978; 37: 742–746.
Tagliabue A, Mantovani A, Kilgallen M, Herberman RB, McCoy JL . Natural cytotoxicity of mouse monocytes and macrophages. J Immunol 1979; 122: 2363–2370.
Perri RT, Kay NE, McCarthy J, Vessella RL, Jacob HS, Furcht LT . Fibronectin enhances in vitro monocyte-macrophage-mediated tomoricidal activity. Blood 1982; 60: 430–435.
Andreesen R, Osterholz J, Bross KJ, Schulz A, Luckenbach GA, Lohr GW . Cytotoxic effector cell function at different stages of human monocyte-macrophage maturation. Cancer Res 1983; 43: 5931–5936.
Burns GF, Triglia T, Bartlett PF, Mackay IR . Human natural killer cells, activated lymphocyte killer cells, and monocytes possess similar cytotoxic mechanisms. Proc Natl Acad Sci USA 1983; 80: 7606–7610.
Ziegler-Heitbrock HWL, Riethmuller G . A rapid assay for cytotoxicity of unstimulated human monocytes. J Natl Cancer Inst 1984; 72: 23–29.
De Maria R, Cifone MG, Trotta R, Rippo MR, Festuccia C, Santoni A et al. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med 1994; 180: 1999–2004.
Zembala M, Uracz W, Ruggiero I, Mytar B, Pryjma J . Isolation and functional characteristics of FcR+ and FcR− human monocyte subsets. J Immunol 1984; 133: 1293–1299.
Hu X, Tang M, Fisher AB, Olashaw N, Zuckerman KS . TNF-alpha-induced growth suppression of CD34+ myeloid leukemic cell lines signals through TNF receptor type I and is associated with NF-kappa B activation. J Immunol 1999; 163: 3106–3115.
Massague J . The transforming growth factor-beta family. Annu Rev Cell Biol 1990; 6: 597–641.
Martin JHJ, Edwards SW . Changes in mechanisms of monocyte/macrophage-mediated cytotoxicity during culture. J Immunol 1993; 150: 3478–3486.
Acknowledgements
We wish to thank Alessandra Mazzoni (Experimental Immunology Branch, NCI, NIH) for helpful discussion and advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a ‘United States Government Work’ paper, as defined by the US Copyright Act
Rights and permissions
About this article
Cite this article
Sconocchia, G., Keyvanfar, K., El Ouriaghli, F. et al. Phenotype and function of a CD56+ peripheral blood monocyte. Leukemia 19, 69–76 (2005). https://doi.org/10.1038/sj.leu.2403550
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403550
Keywords
This article is cited by
-
Molecular and cellular immune features of aged patients with severe COVID-19 pneumonia
Communications Biology (2022)
-
A pilot study on the usefulness of peripheral blood flow cytometry for the diagnosis of lower risk myelodysplastic syndromes: the “MDS thermometer”
BMC Hematology (2018)
-
Monozytäre Subpopulationen in Patienten mit rheumatoider Arthritis
Zeitschrift für Rheumatologie (2017)
-
CD16 is indispensable for antibody-dependent cellular cytotoxicity by human monocytes
Scientific Reports (2016)
-
Origins and tissue‐context‐dependent fates of blood monocytes
Immunology & Cell Biology (2009)