Abstract
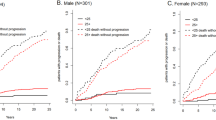
We evaluated the prognostic features of 384 asymptomatic IgM-monoclonal gammopathies (aIgM-MGs) and 74 IgM-related disorders (IgM-RDs), two clinically distinct groups as proposed by the Second International Workshop on Waldenström's Macroglobulinemia (WM). The cumulative probability of evolution to lymphoid malignancy at 5 and 10 years was 8% (95% CI, 5–13%) and 29% (95% CI, 21–38%), respectively, in aIgM-MGs; it was 9% (95% CI, 4–20%) and 16% (95% CI, 7–31%), respectively, in IgM-RDs (P=0.26). At a median follow-up of 45 months (12–233), 45 aIgM-MGs (11.7%) evolved to symptomatic WM (n=41), non-Hodgkin's lymphoma (NHL) (n=2), IgM multiple myeloma (n=1), and primary amyloidosis (n=1). At a median follow-up of 60 months (13–195), seven IgM-RDs (9.5%) evolved to symptomatic WM (n=6), and B-chronic lymphocytic leukaemia (n=1). At univariate analysis, in aIgM-MGs bone marrow lymphoplasmacytic infiltration, high erythrocyte sedimentation rate (ESR), haemoglobin level, IgM size, and lymphocytosis significantly correlated with evolution probability. At multivariate analysis, the latter two parameters strongly correlated with prognosis, haemoglobin being associated with a trend for a higher progression risk. In IgM-RDs IgM size, neutropenia, lymphocytosis, detectable Bence Jones proteinuria, and high ESR were associated with evolution probability. In conclusion, asymptomatic IgM-MGs and IgM-RDs are distinct clinical entities with similar probability of transformation to lymphoid malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gandara DR, Mackenzie MR . Differential diagnosis of monoclonal gammopathy. Med Clin North Am 1988; 72: 1155–1167.
Dhodapkar MV, Jacobson JL, Gertz MA, Crowley JJ, Barlogie B . Prognostic factors and response to fludarabine therapy in patients with Waldenström macroglobulinemia: results of United States intergroup trial (Southwest Oncology Group S9003). Blood 2001; 98: 41–48.
García-Sanz R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A et al. Waldenström macroglobulinemia: presenting features and outcome in a series with 217 cases. Br J Haematol 2001; 115: 575–582.
Facon T, Brouillard M, Duhamel A, Morel P, Simon M, Jouet JP et al. Prognostic factors in Waldenström's macroglobulinemia: a report of 167 cases. J Clin Oncol 1993; 11: 1553–1558.
Morel P, Monconduit M, Jacomy D, Lenain P, Grosbois B, Bateli C et al. Prognostic factors in Waldenström macroglobulinemia: a report on 232 patients with the description of a new scoring system and its validation on 253 other patients. Blood 2000; 96: 852–858.
Gertz MA, Fonseca R, Rajkumar SV . Waldenström's macroglobulinemia. Oncologist 2000; 5: 63–67.
Owen RG, Johnson SA, Morgan GJ . Waldenström's macroglobulinemia: laboratory diagnosis and treatment. Hematol Oncol 2000; 18: 41–49.
Owen RG, Barrans SL, Richards SJ, O'Connor SJ, Child JA, Parapia LA et al. Waldenström macroglobulinemia. Development of diagnostic criteria and identification of prognostic factors. Am J Clin Pathol 2001; 116: 420–428.
Dimopoulos MA, Panayiotidis P, Maulopoulos LA, Sfikakis P, Dalakas M . Waldenström's macroglobulinemia: clinical features, complications, and management. J Clin Oncol 2000; 18: 214–226.
Kyle RA, Garton JP . The spectrum of IgM monoclonal gammopathy in 430 cases. Mayo Clin Proc 1987; 62: 719–731.
Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML et al. Clinicopathological definition of Waldenström's macroglobulinemia: Consensus Panel recommendations from the Second International Workshop on Waldenström's macroglobulinemia. Semin Oncol 2003; 30: 110–115.
Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 2002; 20: 1625–1634.
Baldini L, Guffanti A, Cesana BM, Colombi M, Chiorboli O, Damilano I et al. Role of different hematologic variables in defining the risk of malignant transformation in monoclonal gammopathy. Blood 1996; 87: 912–918.
Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF et al. A long-term study on prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 2002; 346: 564–569.
Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL et al. Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol 1992; 81: 391–394.
Kyle RA . ‘Benign’ monoclonal gammopathy after 20 to 35 years of follow-up. Mayo Clin Proc 1993; 68: 26–36.
Van de Poel MHW, Coebergh JWW, Hillen HFP . Malignant transformation of monoclonal gammopathy of undetermined significance among out-patients of a community hospital of Southeastern Netherlands. Br J Haematol 1995; 91: 121–125.
Dimopoulos MA, Alexanian R . Waldenström's macroglobulinaemia. Blood 1994; 83: 1452–1459.
Zarrabi MH, Stark RS, Kane P, Dannaher CL, Chandor S . IgM myeloma, a distinct entity in the spectrum of B-cell neoplasia. Am J Clin Pathol 1981; 75: 1–10.
Gertz MA, Kyle RA, Noel P . Primary systemic amyloidosis: a rare complication of immunoglobulin M monoclonal gammopathies and Waldenström‘s macroglobulinemia. J Clin Oncol 1993; 11: 914–920.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML et al. A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361–1392.
Vercelli L, Quaglia A, Parodi S, Crosignani P, the ITAPREVAL Working group. Cancer prevalence in the elderly. Tumori 1999; 85: 391–399.
Kyle RA, Treon SP, Alexanian R, Barlogie B, Bjorkholm M, Dhodapkar M et al. Prognostic markers and criteria to initiate therapy in Waldenström's macroglobulinemia: Consensus Panel recommendations from the Second International Workshop on Waldenström's macroglobulinemia. Semin Oncol 2003; 30: 116–120.
Alexanian R, Weber D, Delasalle K, Cabanillas F, Dimopoulos M . Asymptomatic Waldenström's macroglobulinemia. Semin Oncol 2003; 30: 206–210.
Bartl R, Frisch B, Mahl G, Burkhardt R, Fateh-Moghadam A, Pappenberger R et al. Bone marrow histology in Waldenström's macroglobulinaemia. Clinical relevance of subtype recognition. Scand J Haematol 1983; 31: 359–375.
Kyrtsonis MC, Vassilakopoulos TP, Angelopoulou MK, Siakantaris P, Kontopidou FN, Dimopoulou MN et al. Waldenström's macroglobulinemia: clinical course and prognostic factors in 60 patients. Ann Hematol 2001; 80: 722–727.
Gobbi PG, Bettini R, Montecucco C, Cavanna L, Morandi S, Pieresca C et al. Study of prognosis in Waldenström's macroglobulinemia: a proposal for a simple binary classification with clinical and investigational utility. Blood 1994; 83: 2939–2945.
Kyle RA, Therneau TM, Rajkumar SV, Remstein ED, Offord JR, Larson DR et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 2003; 102: 3759–3764.
Pangalis GA, Angelopoulou MK, Vassilakopoulos TP, Siakantaris MP, Kittas C . B-chronic lymphocytic leukemia, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma, including Waldenström‘s macroglobulinemia: a clinical, morphologic, and biologic spectrum of similar disorders. Semin Hematol 1999; 36: 104–114.
Kriangkum J, Taylor BJ, Mant MJ, Treon SP, Belch AR, Pilarski LM . The malignant clone in Waldenström's macroglobulinemia. Semin Oncol 2003; 30: 132–135.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Morra, E., Cesana, C., Klersy, C. et al. Clinical characteristics and factors predicting evolution of asymptomatic IgM monoclonal gammopathies and IgM-related disorders. Leukemia 18, 1512–1517 (2004). https://doi.org/10.1038/sj.leu.2403442
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403442