Abstract
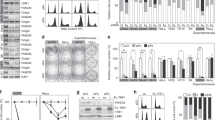
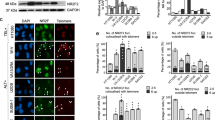
Telomerase activity transiently increases when HL60 cells are treated with the topoisomerase II inhibitor etoposide. A quantitative assessment revealed that telomerase is activated by etoposide treatment in a number of cell lines and that the increase is reversible after withdrawal of etoposide from the cell culture. Telomerase activation correlated with the occurrence of DNA damage but not with cell cycle arrest. We did not detect any transcriptional upregulation of hTERT mRNA, suggesting a post-transcriptional mechanism of telomerase activation. Furthermore, the mRNA expression of the telomere binding protein TRF2 was upregulated early and reversibly after etoposide treatment. TRF1 mRNA expression levels were unchanged after DNA damage, but increased when the cells accumulated in the G2/M phase. The data show that the telosome reacts after DNA damage by upregulating telomerase activity and TRF2 expression in malignant cells. It has previously been shown that overexpression of TRF2 can repress senescence signals arising from critically shortened telomeres. We show here that TRF2 is upregulated by undirected DNA damage that also affects the telomeric DNA. These data suggest that upregulation of telomerase activity and TRF2 expression might act as antiapoptotic mechanisms in the DNA-damage response of malignant cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Collins K . Mammalian telomeres and telomerase. Curr Opin Cell Biol 2000; 12: 378–383.
Blackburn EH . Structure and function of telomeres. Nature 1991; 350: 569–573.
Counter CM, Hirte HW, Bacchetti S, Harley CB . Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci USA 1994; 91: 2900–2904.
Ducrest AL, Szutorisz H, Lingner J, Nabholz M . Regulation of the human telomerase reverse transcriptase gene. Oncogene 2002; 21: 541–552.
Fu W, Begley JG, Killen MW, Mattson MP . Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem 1999; 274: 7264–7271.
Cao Y, Li H, Deb S, Liu JP . TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene 2002; 21: 3130–3138.
Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med 1999; 5: 1164–1170.
Saretzki G, Ludwig A, von Zglinicki T, Runnebaum IB . Ribozyme-mediated telomerase inhibition induces immediate cell loss but not telomere shortening in ovarian cancer cells. Cancer Gene Ther 2001; 8: 827–834.
Lee KH, Rudolph KL, Ju YJ, Greenberg RA, Cannizzaro L, Chin L et al. Telomere dysfunction alters the chemotherapeutic profile of transformed cells. Proc Natl Acad Sci USA 2001; 98: 3381–3386.
Broccoli D, Smogorzewska A, Chong L, De Lange T . Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet 1997; 17: 231–235.
Aragona M, De Divitiis O, La Torre D, Panetta S, D'Avella D, Pontoriero A et al. Immunohistochemical TRF1 expression in human primary intracranial tumors. Anticancer Res 2001; 21: 2135–2139.
De Divitiis O, La Torre D . Inverse correlation of TRF1 expression and cell proliferation in human primary intracranial tumors. J Neurosurg Sci 2001; 45: 1–6.
Matsutani N, Yokozaki H, Tahara E, Tahara H, Kuniyasu H, Kitadai Y et al. Expression of MRE11 complex (MRE11, RAD50, NBS1) and hRap1 and its relation with telomere regulation, telomerase activity in human gastric carcinomas. Pathobiology 2001; 69: 219–224.
Ohyashiki JH, Hayashi S, Yahata N, Iwama H, Ando K, Tauchi T et al. Impaired telomere regulation mechanism by TRF1 (telomere-binding protein), but not TRF2 expression, in acute leukemia cells. Int J Oncol 2001; 18: 593–598.
Yamada K, Yajima T, Yagihashi A, Kobayashi D, Koyanagi Y, Asanuma K et al. Role of human telomerase reverse transcriptase and telomeric-repeat binding factor proteins 1 and 2 in human hematopoietic cells. Jpn J Cancer Res 2000; 91: 1278–1284.
Figueroa R, Lindenmaier H, Hergenhahn M, Nielsen KV, Boukamp P . Telomere erosion varies during in vitro aging of normal human fibroblasts from young and adult donors. Cancer Res 2000; 60: 2770–2774.
Otsuka T, Uchida N, Arima F, Shigematsu H, Fukuyama T, Maeda M et al. Down-regulation of human telomeric protein TRF1 gene expression during myeloid differentiation in human hematopoietic cells. Int J Hematol 2000; 71: 334–339.
van Steensel B, De Lange T . Control of telomere length by the human telomeric protein TRF1. Nature 1997; 385: 740–743.
Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G et al. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 2000; 20: 1659–1668.
Kishi S, Wulf G, Nakamura M, Lu KP . Telomeric protein Pin2/TRF1 induces mitotic entry and apoptosis in cells with short telomeres and is down-regulated in human breast tumors. Oncogene 2001; 20: 1497–1508.
Kishi S, Zhou XZ, Ziv Y, Khoo C, Hill DE, Shiloh Y et al. Telomeric protein Pin2/TRF1 as an important ATM target in response to double strand DNA breaks. J Biol Chem 2001; 276: 29282–29291.
Kishi S, Lu KP . A critical role for Pin2/TRF1 in ATM-dependent regulation. Inhibition of Pin2/TRF1 function complements telomere shortening, radiosensitivity, and the G(2)/M checkpoint defect of ataxia-telangiectasia cells. J Biol Chem 2002; 277: 7420–7429.
Ancelin K, Brunori M, Bauwens S, Koering CE, Brun C, Ricoul M et al. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol Cell Biol 2002; 22: 3474–3487.
van Steensel B, Smogorzewska A, De Lange T . TRF2 protects human telomeres from end-to-end fusions. Cell 1998; 92: 401–413.
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H et al. Mammalian telomeres end in a large duplex loop. Cell 1999; 97: 503–514.
Karlseder J, Broccoli D, Dai Y, Hardy S, De Lange T . p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 1999; 283: 1321–1325.
Karlseder J, Smogorzewska A, De Lange T . Senescence induced by altered telomere state, not telomere loss. Science 2002; 295: 2446–2449.
Moriarty TJ, Dupuis S, Autexier C . Rapid upregulation of telomerase activity in human leukemia HL-60 cells treated with clinical doses of the DNA-damaging drug etoposide. Leukemia 2002; 16: 1112–1120.
Sato N, Mizumoto K, Kusumoto M, Nishio S, Maehara N, Urashima T et al. Up-regulation of telomerase activity in human pancreatic cancer cells after exposure to etoposide. Br J Cancer 2000; 82: 1819–1826.
Lin Z, Lim S, Viani MA, Sapp M, Lim MS . Down-regulation of telomerase activity in malignant lymphomas by radiation and chemotherapeutic agents. Am J Pathol 2001; 159: 711–719.
Krupp G, Kuhne K, Tamm S, Klapper W, Heidorn K, Rott A et al. Molecular basis of artifacts in the detection of telomerase activity and a modified primer for a more robust ‘TRAP’ assay. Nucleic Acids Res 1997; 25: 919–921.
Klapper W, Singh KK, Heidorn K, Parwaresch R, Krupp G . Regulation of telomerase activity in quiescent immortalized human cells. Biochim Biophys Acta 1998; 1442: 120–126.
Wersto RP, Chrest FJ, Leary JF, Morris C, Stetler-Stevenson MA, Gabrielson E . Doublet discrimination in DNA cell-cycle analysis. Cytometry 2001; 46: 296–306.
Blackburn EH . Switching and signaling at the telomere. Cell 2001; 106: 661–673.
Tsujimoto H, Usami N, Hasegawa K, Yamada T, Nagaki K, Sasakuma T . De novo synthesis of telomere sequences at the healed breakpoints of wheat deletion chromosomes. Mol Gen Genet 1999; 262: 851–856.
Diede SJ, Gottschling DE . Telomerase-mediated telomere addition in vivo requires DNA primase and DNA polymerases alpha and delta. Cell 1999; 99: 723–733.
Bottius E, Bakhsis N, Scherf A . Plasmodium falciparum telomerase: de novo telomere addition to telomeric and nontelomeric sequences and role in chromosome healing. Mol Cell Biol 1998; 18: 919–925.
Wang H, Blackburn EH . De novo telomere addition by Tetrahymena telomerase in vitro. EMBO J 1997; 16: 866–879.
Morin GB . Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature 1991; 353: 454–456.
Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J 2001; 20: 6958–6968.
Gieseler F, Bauer E, Nuessler V, Clark M, Valsamas S . Molecular effects of topoisomerase II inhibitors in AML cell lines: correlation of apoptosis with topoisomerase II activity but not with DNA damage. Leukemia 1999; 13: 1859–1863.
Yoon HJ, Choi IY, Kang MR, Kim SS, Muller MT, Spitzner JR et al. DNA topoisomerase II cleavage of telomeres in vitro and in vivo. Biochim Biophys Acta 1998; 1395: 110–120.
Faraoni I, Turriziani M, Masci G, De Vecchis L, Shay J W, Bonmassar E et al. Decline in telomerase activity as a measure of tumor cell killing by antineoplastic agents in vitro. Clin Cancer Res 1997; 3: 579–585.
Sawant SG, Gregoire V, Dhar S, Umbricht CB, Cvilic S, Sukumar S et al. Telomerase activity as a measure for monitoring radiocurability of tumor cells. FASEB J 1999; 13: 1047–1054.
Akiyama M, Horiguchi-Yamada J, Saito S, Hoshi Y, Yamada O, Mizoguchi H et al. Cytostatic concentrations of anticancer agents do not affect telomerase activity of leukaemic cells in vitro. Eur J Cancer 1999; 35: 309–315.
Nakagami Y, Ito M, Hara T, Inoue T . Loss of TRF2 by radiation-induced apoptosis in HL60 cells. Radial Med 2002; 20: 121–129.
Multani AS, Ozen M, Narayan S, Kumar V, Chandra J, McConkey DJ et al. Caspase-dependent apoptosis induced by telomere cleavage and TRF2 loss. Neoplasia 2000; 2: 339–345.
Narayan S, Jaiswal AS, Multani AS, Pathak S . DNA damage-induced cell cycle checkpoints involve both p53-dependent and -independent pathways: role of telomere repeat binding factor 2. Br J Cancer 2001; 85: 898–901.
Acknowledgements
We thank M Hauberg for her help with immunohistochemistry and E Dege for critically reading the manuscript. This work was supported in part by the ‘Kinder Krebs Initiative Buchholz-Seppensen-Holm’, Germany.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klapper, W., Qian, W., Schulte, C. et al. DNA damage transiently increases TRF2 mRNA expression and telomerase activity. Leukemia 17, 2007–2015 (2003). https://doi.org/10.1038/sj.leu.2403086
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403086
Keywords
This article is cited by
-
Non-canonical roles of canonical telomere binding proteins in cancers
Cellular and Molecular Life Sciences (2021)
-
Telomeric impact of conventional chemotherapy
Frontiers of Medicine (2013)
-
TRF2 inhibition promotes anchorage-independent growth of telomerase-positive human fibroblasts
Oncogene (2006)
-
Accumulation and altered localization of telomere-associated protein TRF2 in immortally transformed and tumor-derived human breast cells
Oncogene (2005)
-
AtTBP2 and AtTRP2 in Arabidopsis encode proteins that bind plant telomeric DNA and induce DNA bending in vitro
Molecular Genetics and Genomics (2005)