Abstract
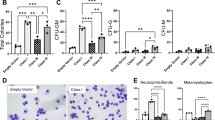
WT1 is expressed in hematopoietic progenitor cells and in acute leukemia, but its role in normal and malignant hematopoiesis has not been clearly defined. Alternative splicing of the WT1 mRNA yields several protein isoforms with distinct DNA binding and transcriptional regulatory activities. In this study, we investigated the effect of the WT1 isoform lacking two alternatively spliced sequences (WT1 (−/−)) in 32D cl3 cells, a murine myeloid progenitor cell line. The expression of WT1 (−/−) accelerated the granulocyte-colony stimulating factor (G-CSF)-mediated differentiation of these cells, as judged by morphology and by the expression of differentiation-associated genes and cell surface antigens. WT1 (−/−) inhibited G1/S progression in G-CSF but not in interleukin-3, potentially accounting for its ability to accelerate differentiation. It is likely that dominant-negative mutants previously reported in leukemia patients participate in leukemogenesis by inhibiting this function of the wild-type protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 1990; 60: 509–520.
Gessler M, Poustka A, Cavenee W, Neve RL, Orkin SH, Bruns GAP . Homozygous deletion in Wilms' tumor of a zinc-finger gene identified by chromosome jumping. Nature 1990; 343: 774–778.
Sharma PM, Bowman M, Madden SL, Rauscher III FJ, Sukumar S . RNA editing in the Wilms' tumor susceptibility gene, WT1. Genes Dev 1994; 8: 720–731.
Bickmore WA, Oghene K, Little MH, Seawright A, van Heyningen V, Hastie ND . Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science 1992; 257: 235–237.
Wang ZY, Qiu QQ, Huang J, Gurrieri M, Deuel TF . Products of alternatively spliced transcripts of the Wilms' tumor suppressor gene, wt1, have altered DNA binding specificity and regulate transcription in different ways. Oncogene 1995; 10: 415–422.
Drummond IA, Rupprecht HD, Rohwer-Nutter P, Lopez-Guisa JM, Madden SL, Rauscher III FJ et al. DNA recognition by splicing variants of the Wilms' tumor suppressor, WT1. Mol Cell Biol 1994; 14: 3800–3809.
Little MH, Holmes G, Pell L, Caricasole A, Duarte A, Law M et al. A novel target for the Wilms' tumour suppressor protein (WT1) is bound by a unique combination of zinc fingers. Oncogene 1996; 13: 1461–1469.
Larsson SH, Charlieu JP, Miyagawa K, Engelkamp D, Rassoulzadegan M, Ross A et al. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell 1995; 81: 391–401.
Maurer U, Brieger J, Weidmann E, Mitrou PS, Hoelzer D, Bergmann L . The Wilms' tumor gene is expressed in a subset of CD34+ progenitors and downregulated early in the course of differentiation in vitro. Exp Hematol 1997; 25: 945–950.
Baird PN, Simmons PJ . Expression of the Wilms' tumor gene (WT1) in normal hemopoiesis [see comments]. Exp Hematol 1997; 25: 312–320.
Phelan SA, Lindberg C, Call KM . Wilms' tumor gene, WT1, mRNA is downregulated during induction of erythroid and megakaryocytic differentiation of K562 cells. Cell Growth Differ 1994; 5: 677–686.
Sekiya M, Adachi M, Hinoda Y, Imai K, Yachi A . Downregulation of Wilms' tumor gene (wt1) during myelomonocytic differentiation in HL60 cells. Blood 1994; 83: 1876–1882.
Svedberg H, Chylicki K, Gullberg U . Downregulation of Wilms' tumor gene (WT1) is not a prerequisite for erythroid or megakaryocytic differentiation of the leukemic cell line K562. Exp Hematol 1999; 27: 1057–1062.
Svedberg H, Chylicki K, Baldetorp B, Rauscher III FJ, Gullberg U . Constitutive expression of the Wilms' tumor gene (WT1) in the leukemic cell line U937 blocks parts of the differentiation program. Oncogene 1998; 16: 925–932.
Ellisen LW, Carlesso N, Cheng T, Scadden DT, Haber DA . The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells. Embo J 2001; 20: 1897–1909.
Smith SI, Weil D, Johnson GR, Boyd AW, Li CL . Expression of the Wilms' tumor suppressor gene, WT1, is upregulated by leukemia inhibitory factor and induces monocytic differentiation in M1 leukemic cells. Blood 1998; 91: 764–773.
Valtieri M, Tweardy DJ, Caraciollo D, Johnson K, Mavilio F, Altman S et al. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J Immunol 1987; 138: 3829–3835.
Greenberger JS, Ann Sakakeeny M, Keith Humphries R, Eaves CJ, Eckner RJ . Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc Natl Acad Sci USA 1983; 80: 2931–2935.
Inoue K, Tamaki H, Ogawa H, Oka Y, Soma T, Tatekawa T et al. Wilms' tumor gene (WT1) competes with differentiation-inducing signal in hematopoietic progenitor cells. Blood 1998; 91: 2969–2976.
Friedman AD, Krieder BL, Venturelli D, Rovera G . Transcriptional regulation of two myeloid-specific genes, myeloperoxidase and lactoferrin, during differentiation of the murine cell line 32D C13. Blood 1991; 78: 2426–2432.
Barbaux S, Niaudet P, Gubler MC, Grunfeld JP, Jaubert F, Kuttenn F et al. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 1997; 17: 467–470.
Klamt B, Koziell A, Poulat F, Wieacker P, Scambler P, Berta P et al. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/−KTS splice isoforms. Hum Mol Genet 1998; 7: 709–714.
Renshaw J, King-Underwood L, Pritchard-Jones K . Differential splicing of exon 5 of the Wilms' tumour (WT1) gene. Genes Chromosomes Cancer 1997; 19: 256–266.
King-Underwood L, Renshaw J, Pritchard-Jones K . Mutations in the Wilms' tumor gene WT1 in leukemias. Blood 1996; 87: 2171–2179.
King-Underwood L, Pritchard-Jones K . Wilms' tumor (WT1) gene mutations occur mainly in acute myeloid leukemia and may confer drug resistance. Blood 1998; 91: 2961–2968.
Loeb DM, Korz D, Katsnelson M, Burwell EA, Friedman AD, Sukumar S . Cyclin E is a target of WT1 transcriptional repression. J Biol Chem 2002; 277: 19627–19632.
Englert C, Maheswaran S, Garvin AJ, Kreidberg J, Haber DA . Induction of p21 by the Wilms' tumor suppressor gene WT1. Cancer Res 1997; 57: 1429–1434.
Wang X, Scott E, Sawyers CL, Friedman AD . C/EBPalpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood 1999; 94: 560–571.
Ward AC, Loeb DM, Soede-Bobok AA, Touw IP, Friedman AD . Regulation of granulopoiesis by transcription factors and cytokine signals. Leukemia 2000; 14: 973–990.
Bies J, Mukhopadhyaya R, Pierce J, Wolff L . Only late, nonmitotic stages of granulocyte differentiation in 32Dcl3 cells are blocked by ectopic expression of murine c-myb and its truncated forms. Cell Growth Differ 1995; 6: 59–68.
Acknowledgements
This work was supported by a grant from the National Institutes of Health, R01-CA48943 to SS. ADF is a Scholar of the Leukemia and Lymphoma Society. DML is a Research Fellow of the Leukemia and Lymphoma Society and received grants from the Lauri Strauss Leukemia Foundation and the Bear Necessities Pediatric Cancer Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Loeb, D., Summers, J., Burwell, E. et al. An isoform of the Wilms' tumor suppressor gene potentiates granulocytic differentiation. Leukemia 17, 965–971 (2003). https://doi.org/10.1038/sj.leu.2402906
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402906
Keywords
This article is cited by
-
Acquired WT1 mutations contribute to relapse of NPM1-mutated acute myeloid leukemia following allogeneic hematopoietic stem cell transplant
Bone Marrow Transplantation (2022)
-
Tumour-associated antigens: considerations for their use in tumour immunotherapy
International Journal of Hematology (2011)
-
Isoforms of Wilms’ tumor suppressor gene (WT1) have distinct effects on mammary epithelial cells
Oncogene (2007)
-
Wilms' tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring
Leukemia (2006)
-
Antiapoptotic function of 17AA(+)WT1 (Wilms' tumor gene) isoforms on the intrinsic apoptosis pathway
Oncogene (2006)