Abstract
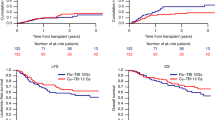
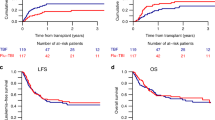
In order to improve the disappointing prognosis of adult patients with acute lymphoblastic leukemia (ALL), we applied similar induction therapy as that used for acute myeloid leukemia (AML), ie frequent administration of doxorubicin (DOX). DOX 30 mg/m2 was administered from days 1 to 3 and from days 8 to 10 together with vincristine, prednisolone, cyclophosphamide and L-asparaginase, followed by three courses of consolidation and four courses of intensification. From December 1993 to February 1997, 285 untreated adult patients with de novo ALL were entered. Of 263 evaluable patients (age 15 to 59; median 31), 205 (78%) obtained complete remission (CR). At a median follow-up period of 63 months, the predicted 6-year overall survival (OS) rate of all patients was 33%, and disease-free survival (DFS) rate of CR patients was 30%, respectively. By multivariate analysis, favorable prognostic factors for the achievement of CR were age <40 and WBC <50 000/μl; for longer OS were age <30 and WBC <30 000/μl; and for longer DFS of CR patients were FAB L1 and ALT <50 IU/l. Among 229 patients who had adequate cytogenetic data, 51 (22%) had Philadelphia (Ph) chromosome. Ph-negative chromosome was a common favorable prognostic factor for CR, longer OS and DFS. DFS was not different between early sequential intensification (n = 48) and intermittent intensification (n = 43) during the maintenance phase. Among CR patients under 40 years old, the 6-year survival was not different between the allocated related allo-BMT group (34 patients) and the allocated chemotherapy group (108 patients). However, among patients with Ph-positive ALL, the survival of patients who actually received allo-BMT was superior to that of patients who received chemotherapy (P = 0.046).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Koizumi S, Fujimoto T, Oka T, Watanabe S, Kikuta A, Tshuchiya T, Matsushita T, Yanase T, Mimaya J, Ohta S, Miyake M, Nishikawa K, Furuyama T, Yamamura Y, Takaue Y, Ninomiya T, Shimokawa T, Iwai A, Ishida Y, Ariyoshi N, Kimura K, Kawakami K, Gushiken T, Sekine I . Overview of clinical studies of childhood acute lymphoblastic leukemia for more than ten years by the Japanese Children's Cancer and Leukemia Study Group Pediatr Hematol Oncol 1997 14: 17–28
Pui C . Acute lymphoblastic leukemia N Engl J Med 1998 339: 605–615
Kantarjian HM, Walters RS, Keating MJ, Smith TL, O'Brien S, Estey EH, Huh YO, Spinolo J, Dicke K, Barlogie B, McCredie KB, Freireich EJ . Results of the vincristine, doxorubicin, and dexamethasone regimen in adults with standard- and high-risk acute lymphocytic leukemia J Clin Oncol 1990 8: 994–1004
Linker CA, Levitt LJ, O'Donnell M, Forman SJ, Ries CA . Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report Blood 1991 78: 2814–2822
Hoelzer D, Thiel E, Ludwig WD, Loffler H, Buchner T, Freund M, Heil G, Hiddemann W, Maschmeyer G, Volkers B, Gokbuget N, Aydemir U for the German Adult ALL Study Group. Follow-up of the first two successive German multicentre trials for adult ALL (01/81 and 02/84). German Adult ALL Study Group Leukemia 1993 7 (Suppl. 2): S130–134
Mandelli F, Annino L, Rotoli B for the GIMEMA Cooperative Group. The GIMEMA ALL 0183 trial: analysis of 10-year follow-up Br J Haematol 1996 92: 665–672
Durrant IJ, Prentice HG, Richards SM . Intensification of treatment for adults with acute lymphoblastic leukaemia: Results of U.K. Medical Research Council randomized trial UKALL XA Br J Haematol 1997 99: 84–92
Larson R . Recent clinical trials in acute lymphocytic leukemia by the Cancer and Leukemia Group B Hematol Oncol Clin North Am 2000 14: 1367–1379
Tanimoto M, Miyawaki S, Ino T, Kyo T, Sakamaki H, Naoe T, Hiraoka A, Asou N, Ohshima T, Tsubaki K, Kuriyama K, Ueda T, Minami S, Okabe K, Saito H, Murakami H, Hirano M, Dohy H, Onozawa Y, Suzuki H, Ohno R . Response-oriented individualized induction therapy followed by intensive consolidation and maintenance for adult patients with acute lymphoblastic leukemia: the ALL-87 study of Japan Adult Leukemia Study Group (JALSG) Int J Hematol 1998 68: 421–429
Ueda T, Miyawaki S, Asou N, Kuraishi Y, Hiraoka A, Kuriyama K, Minami S, Ohshima T, Ino T, Tamura J, Kanamaru A, Nishikawa K, Tanimoto M, Oh H, Saito K, Nagata K, Naoe T, Yamada O, Urasaki Y, Sakura T, Ohno R . Response-oriented individualized induction therapy with 6 drugs followed by 4 courses of intensive consolidation, one-year maintenance and intensification therapy: the ALL90 study of the Japan Adult Leukemia Study Group Int J Hematol 1998 68: 279–289
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton AG, Gralnick HR, Sultan C the French–American–British (FAB) Co-operative Group. Proposals for the classification of acute leukaemias Br J Haematol 1976 33: 626–629
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton AG, Gralnick HR, Sultan C the French–American–British (FAB) Co-operative Group. The morphological classification of acute lymphoblastic leukaemia: concordance among observers and clinical correlations Br J Haematol 1981 47: 553–561
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton AG, Gralnick HR, Sultan C . Criteria for the diagnosis of acute leukemia of megakaryocyte lineage (M7) Ann Intern Med 1985 103: 460–462
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton AG, Gralnick HR, Sultan C the French–American–British (FAB) Co-operative Group. Proposals for the recognition of minimally differentiated acute myeloid leukaemia (AML-M0) Br J Haematol 1991 78: 325–329
Todeschini G, Tecchio C, Meneghini V, Pizzolo G, Veneri D, Zanotti R, Ricetti MM, Solero P, Aprili F, Perona G . Estimated 6-year event-free survival of 55% in 60 consecutive adult acute lymphoblastic leukemia patients treated with an intensive phase II protocol based on high induction dose of daunorubicin Leukemia 1998 12: 144–149
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating M, Murphy S, Freireich EJ . Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia J Clin Oncol 2000 18: 547–561
Durrant IJ, Richards SM, Prentice HG, Goldstone AH . The Medical Research Council trials in adult acute lymphocytic leukemia Hematol Oncol Clin North Am 2000 14: 1327–1352
Hoelzer D, Thiel E, Loffler H, Buchner T, Ganser A, Heil G, Koch P, Freund M, Diedrich H, Ruhl H, Maschmeyer G, Lipp T, Nowrousian MR, Burkert M, Gerecke D, Pralle H, Muller U, Lunscken Ch, Fulle H, Ho AD, Kuchler R, Busch FW, Schneider W, Gorg Ch, Emmerich B, Braunmann D, Vaupel HA, von Paleske A, Bartels H, Neiss A, Messerner D . Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults Blood 1988 71: 123–131
Verma A, Stock W . Management of adult acute lymphoblastic leukemia: moving toward a risk-adapted approach Curr Opin Oncol 2001 13: 14–20
Czuczman MS, Dodge RK, Stewart CC, Frankel SR, Davey FR, Powell BL, Szatrowski TP, Schiffer CA, Larson RA, Bloomfield CD . Value of immunophenotype in intensively treated adult acute lymphoblastic leukemia: Cancer and Leukemia Group B study Blood 1999 93: 3931–3939
Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, Odenwald E, Ritter J, Mann G, Welte K, Gadner H, Riehm H . Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86 Blood 1994 84: 3122–3133
Rivera G, Raimondi S, Hancock M, Behm F, Pui CH, Abromowitch M, Mirro J, Ochs J, Look A, Williams D, Murphy S, Dahl G, Kalwinsky D, Evans W, Kun L, Simone J, Crist W . Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy Lancet 1991 337: 61–66
Hoelzer D, Ludwig WD, Thiel E, Gassmann W, Loffler H, Fonatsch C, Rieder H, Heil G, Heinze B, Arnold R, Hossfeld D, Buchner T, Koch P, Freund M, Hiddemann W, Maschmeyer G, Heyll A, Aul C, Faak T, Kuse R, Ittel TH, Gramatzki M, Diedrich H, Kolbe K, Fuhr HG, Fischer K, Schadeck-Gressel C, Weiss A, Strohscheer I, Metzner B, Fabry U, Gokbuget N, Volkers B, Messerner D, Uberla K . Improved outcome in adult B-cell acute lymphoblastic leukemia Blood 1996 87: 495–508
The Japan Society for Hematopoietic Cell Transplantation. Annual Report of Nationwide Survey 2000 The Japan Society for Hematopoietic Cell Transplantation Office of Nationwide Survey: Nagoya 2000
Oh H, Gale RP, Zhang MJ, Passweg JR, Ino T, Murakami H, Ohno R, Rowlings PA, Sobocinski KA, Tanimoto M, Tomonaga M, Weisdorf DJ, Horowitz MM . Chemotherapy vs HLA-identical sibling bone marrow transplants for adults with acute lymphoblastic leukemia in first remission Bone Marrow Transplant 1998 22: 253–257
Faderl S, Kantarjian HM, Thomas DA, Cortes J, Giles F, Pierce S, Albitar M, Estrov Z . Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia Leuk Lymphoma 2000 36: 263–273
Ottmann OG, Sawyers C, Drucker B, Reiffers J, Goldman JM, O'Brien SG, Reese SF, Cpdeville R The International STI571 Study Group. A phase II study to determine the safety and antileukemic effects of STI571 in adult patients with Philadelphia chromosome positive acute leukemias Blood 2000 96: 828a
Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M . Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blastic crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with Philadelphia chromosome New Engl J Med 2001 344: 1038–1042
Acknowledgements
The authors are grateful to all participating physicians from 40 institutions in the Japan Adult Leukemia Study Group for their cooperation with this study. Thanks also to Ms Yuko Makino for her excellent secretarial assistance. This study was supported, in part, by Grants-in-Aid for Cancer Research 9–2 from the Ministry of Health and Welfare, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takeuchi, J., Kyo, T., Naito, K. et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia 16, 1259–1266 (2002). https://doi.org/10.1038/sj.leu.2402526
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2402526
Keywords
This article is cited by
-
Propensity score matching/reweighting analysis comparing autologous and allogeneic stem cell transplantation for B-lineage acute lymphoblastic leukemia
International Journal of Hematology (2022)
-
Optimal treatment for Philadelphia-negative acute lymphoblastic leukemia in first remission in the era of high-intensity chemotherapy
International Journal of Hematology (2021)
-
Current Approaches to Philadelphia Chromosome–Positive B-Cell Lineage Acute Lymphoblastic Leukemia: Role of Tyrosine Kinase Inhibitor and Stem Cell Transplant
Current Oncology Reports (2021)
-
The impacts of BCR-ABL1 mutations in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia who underwent allogeneic hematopoietic cell transplantation
Annals of Hematology (2020)
-
High-dose methotrexate therapy significantly improved survival of adult acute lymphoblastic leukemia: a phase III study by JALSG
Leukemia (2018)