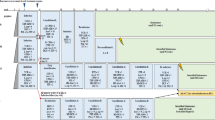
Abstract
In this population-based material from the five Nordic countries (Denmark, Finland, Iceland, Norway and Sweden), 2860 children below 15 years of age were diagnosed with acute lymphoblastic leukemia (ALL) from July 1981 to June 1998. The annual incidence was 3.9/100 000 children and was stable throughout the study period. The development from regional or national protocols to common Nordic treatment protocols for all risk groups was completed in 1992 through a successive intensification with multidrug chemotherapy, including pulses of methotrexate in high doses and avoidance of cranial irradiation in most children. The overall event-free survival (EFS) at 5 years has increased from 56.5 ± 1.7% in the early 1980s to 77.6 ± 1.4% during the 1990s. The main improvements were seen in children with non-high risk leukemia. In high-risk patients, progress has been moderate, especially in children with high WBC (⩾100 × 109/l) at diagnosis. During the last time period (January 1992–June 1998), only 10% of the patients have received cranial irradiation in first remission, while 90% of the patients have received pulses of high dose methotrexate (5–8 g/m2) isolated or combined with high-dose cytosine arabinoside (total dose 12 g/m2) plus multiple intrathecal injections of methotrexate as CNS-targeted treatment, not translating into increased cumulative incidence of CNS relapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pinkel D, Simone J, Hustu O, Auer RJA . Nine years experience with ‘total therapy’ of childhood acute lymphoblastic leukaemia Pediatrics 1972 50: 246–290
Riehm H, Gadner H, Henze G, Kornhuber B, Lampert F, Niethammar D, Reiter A, Schellong G . Results and significance of six randomised trials in four consecutive ALL-BFM studies Hamatol Bluttransfus 1990 33: 439–450
Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D . Chemotherapy in 998 unselected childhood acute lymphoblastic leukaemia patients. Results and conclusions of the multicenter trial ALL-BFM 86 Blood 1994 84: 3122–3133
Pui CH . Childhood leukaemia New Engl J Med 1995 332: 1618–1630
Gustafsson G, Kreuger A, Clausen N, Garwicz S, Kristinsson J, Lie SO, Moe PJ, Perkkiö M, Yssing M, Saarinen-Pihkala UM on behalf of NOPHO . Intensified treatment of acute lymphoblastic childhood leukaemia has improved prognosis, especially in non-high risk patients. The Nordic experience of 2648 patients diagnosed between 1981 and 1996 Acta Paediatrica 1998 87: 1151–1161
Gustafsson G, Garwicz S, Hertz H, Johannesson G, Jonmundsson G, Moe PJ, Salmi T, Seip M, Siimes MA, Yssing M, Ahstrom L for NOPHO . A population-based study of childhood acute lymphoblastic leukaemia diagnosed from July 1981 through June 1985 in the five Nordic countries Acta Paediatr Scand 1987 76: 781–788
Wollner N, Exilby PR, Liebermann P . Non-Hodgkin's lymphoma in children Cancer 1979 44: 1990–1999
Schmiegelow K, Schröder H, Gustafsson G, Kristiansson J, Glomstein A, Salmi T, Wranne L . Risk of relapse in childhood acute lymphoblastic leukemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. Nordic Society of Pediatric Hematology and Oncology J Clin Oncol 1995 13: 345–351
Nourusis MJ . SPSS statistical software. SPSS: Base and Advanced Statistics 10.0 SPSS Inc: Chicago 1999
Gustafsson G, Berglund G, Garwicz S, Hertz H, Jonmundsson G, Moe PJ, Salmi T, Seip M, Siimes MA, Yssing M, Ahstrom L for NOPHO . A population-based study of children with standard risk acute lymphoblastic leukaemia in the five Nordic countries Acta Paediatr Scand 1989 78: 104–109
Schmiegelow K, Glomstein A, Kristinsson J, Salmi T, Bjork O . Impact of morning versus evening schedule for oral methotrexate and 6-mercaptopurine on relapse risk for children with acute lymphoblastic leukemia. Nordic Society of Pediatric Hematology and Oncology (NOPHO) J Pediatr Hematol Oncol 1997 19: 102–109
Moe PJ, Seip M, Finne PH . Intermediate dose methotrexate in childhood acute lymphocytic leukemia Acta Paediatr Scand 1981 70: 73–79
Moe PJ . Recent advances in the management of acute lymphocytic leukaemia Eur Paediatr Haematol Oncol 1984 1: 19–22
Moe PJ, Wesenberg F, Kolmannskog S . Methotrexate infusions in poor prognosis acute lymphocytic leukaemia: II. High dose methotrexate (HDM) in acute lymphocytic leukaemia in childhood. A pilot study from 1981 Med Pediatr Oncol 1986 14: 189–190
Saarinen U, Mellander L, Nystrom K, Ringden O, Schroeder H, Glomstein A, Gustafsson G . Allogeneic bone marrow transplantation in first remission for children with very high-risk acute lymphoblastic leukaemia: a retrospective case–control study in the Nordic countries Bone Marrow Transplant 1996 17: 357–363
Schrappe M, Reiter A, Ludwig WF, Harbott J, Zimmermann M, Hiddemann W, Niemeyer C, Henze G, Feldyes A, Zintl F, Kornhuber B, Ritter J, Welte K, Gadner H, Riehm H . Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90 Blood 2000 95: 3310–3322
Lilleyman JS, Lennard L . Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukemia Lancet 1994 343: 1188–1190
Forestier E, Johansson B, Gustafsson G, Borgström G, Kerndrup G, Johansson J, Heim S . Prognostic impact of karyotypic findings in childhood acute lymphoblastic leukemia: a Nordic series comparing two treatment periods Br J Haematol 2000 110: 147–153
Kaspers GJ, Veerman AJ, Pieters R, Van Zantwijk CH, Smets LA, Van Wering ER, Van Der Does-Van Den Berg A . In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukaemia Blood 1997 90: 2723–2729
van Dongen JJ, Seriu T, Panzer Grumayer ER, Biondi A, Pongers WM, Corral L, Stolz F, Schrappe M, Masera G, Kamps W-A, Gadner H, Van Wering ER, Ludwig WD, Basso G, de Bruijn MA, Cazzaniga G, Hettinger K, Van Der Does-Van Den Berg A, Hop WC, Riehm H, Bartram C . Prognostic value of minimal residual disease in acute lymphoblastic leukemia in childhood Lancet 1998 352: 1731–1738
Coustan-Smith E, Behm FG, Sanchez J, Boyett JM, Hanook ML, Raimondi SC, Rubnitz JE, Rivera GK, Sandlund JT, Pui CH, Campana D . Immunological detection of minimal residual disease in children with acute lymphoblastic leukemia Lancet 1998 351: 550–554
Acknowledgements
Financial support was received from the Swedish Child Cancer Foundation and the Cancer Foundations in Denmark, Norway and Finland.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Gustafsson, G., Schmiegelow, K., Forestier, E. et al. Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of CNS irradiation. Leukemia 14, 2267–2275 (2000). https://doi.org/10.1038/sj.leu.2401961
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401961