Abstract
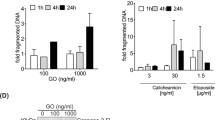
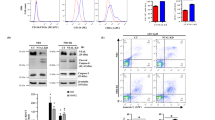
Arsenic trioxide (As2O3)-treatment is effective in acute promyelocytic leukemia (APL) patients with t(15;17). Clinically achievable concentrations of As2O3 induce apoptosis in NB4, an APL cell line, in vitro. Here, to study the mechanism of As2O3-induced apoptosis, we established an As2O3-resistant subline, NB4/As. Growth of NB4/As was inhibited by 50% after 2 day-treatment (IC50) at 1.6 μM As2O3, whereas IC50 of NB4 was 0.3 μM. Degradation of PML-RARα and change of the PML-subcellular localization were similarly induced by As2O3 in NB4 and NB4/As, suggesting that their contribution to apoptosis is small. Treatment with 1 μM As2O3 induced the activation of caspase 3 as well as a loss of mitochondrial transmembrane potential (ΔΨm) in NB4 but not in NB4/As. Caspase 8 and Bid were also activated by As2O3 in NB4 but not in NB4/As. In NB4, an inhibitor of caspase 8 blocked not only the activation of caspase 3 but also the loss of ΔΨm. Neither cell line expressed CD95/Fas, and agonistic anti-Fas antibody (CH-11) failed to cause apoptosis. Neither antagonistic anti-CD95/Fas antibody nor anti-Fas ligand antibodies influenced the As2O3-induced apoptosis. NB4/As had a higher concentration of intracellular glutathione (GSH) than NB4 (96 vs 32 nmol/mg). Reduction of the GSH level by buthionine sulfoxide (BSO) completely restored the sensitivity to As2O3 in NB4/As. Furthermore, caspase activation and the loss of ΔΨm were recovered by combination treatment with BSO. These findings suggest that the As2O3 treatment activates caspase 8 in a CD95-independent but GSH concentration-dependent manner. In combination with BSO, As2O3 might be applied to therapy of leukemia/cancers which are insensitive to the clinically achievable concentrations of As2O3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Zhang P, Wang SY, Hu XH . Arsenic trioxide treated 72 cases of acute promyelocytic leukemia Clin J Hematol 1996 17: 58–61
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY . Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy andpharmacokinetics in relapsed patients Blood 1997 89: 3354–3360
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP . Warrell RP. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide New Engl J Med 1998 339: 1341–1348
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z . In vitro studies on cellular and molecular mechanisms of arsenic trioxide(As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RARα/PML proteins Blood 1996 88: 1052–1061
Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de Thé H, Chen SJ, Chen Z . Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells Blood 1997 89: 3345–3353
Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo Coco F, Avvisati G, Testa U, Peschle C, Gambacorti-Passerini C, Nervi C, Miller WH . Arsenic trioxide as an inducer of apoptosis and loss of PML/RARα protein in acute promyelocytic leukemia cells J Natl Cancer Inst 1998 90: 124–133
Kitamura K, Yoshida H, Ohno R, Naoe T . Toxic effects of arsenic (As3+) and other metal ions on acute promyelocytic leukemic cells Int J Hematol 1997 65: 179–185
de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A . The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR Cell 1991 66: 675–684
Kakizuka A, Miller WJ, Umesono K, Warrell RJ, Frankel SR, Murty VV, Dmitrovsky E, Evans RM . Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML Cell 1991 66: 663–674
Koken MHM, Daniel M-T, Gianni M, Zelent A, Licht J, Buzyn A, Minard P, Degos L, Varet B, de Thé H . Retinoic acid, but not arsenic trioxide, degrades the PLZF/RARα fusion protein, without inducing terminal differentiation or apoptosis, in a RA-therapy resistant t(11;17)(q23;q21) APL patient Oncogene 1999 18: 1113–1118
Zhu J, Koken MHM, Quignon F, Chelbi-Alix M, Degos L, Wang ZY, Chen Z, de Thé H . Arsenic-induced PML targeting onto nuclear bodies: implications for the treatment of acute promyelocytic leulemia Proc Natl Acad Sci USA 1997 94: 3978–3983
Muller S, Matunis MJ, Dejean A . Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus EMBO J 1998 17: 61–70
Sternsdorf T, Puccetti E, Jensen K, Hoelzer D, Will H, Ottmann OG, Ruthardt M . PIC-1/SUMO-1-modified PML-retinoic acid receptor α mediates arsenic trioxide-induced apoptosis in acute promyelocytic leukemia Mol Cell Biol 1999 19: 5170–5178
Quignon F, Chen Z, de Thé H . Retinoic acid and arsenic: towards oncogene-targeted treatments of acute promyelocytic leukemia Biochim Biophys Acta 1997 1333: M53–M61
Look AT . Arsenic and apoptosis in the treatment of acute promyelocytic leukemia J Natl Cancer Inst 1998 90: 86–88
Lallemand-Breitenbach V, Guillemin M-C, Janin A, Daniel M-T, Degos L, Kogan SC, Bishop JM, de Thé H . Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia J Exp Med 1999 189: 1043–1052
Akao Y, Mizoguchi H, Kojima S, Naoe T, Ohishi N, Yagi K . Arsenic induces apoptosis in B-cell leukaemic cell lines in vitro: activation of caspases and down-regulation of Bcl-2 protein Br J Haematol 1998 102: 1055–1060
Wang ZG, Rivi R, Delva L, Konig A, Scheinberg DA, Gambacorti-Passerini C, Gabrilove JL, Warrell RP, Pandolfi PP . Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARα independent manner Blood 1998 92: 1497–1504
Zhang W, Ohnishi K, Shigeno K, Fujisawa S, Naito K, Nakamura S, Takeshita K, Takeshita A, Ohno R . The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms Leukemia 1998 12: 1383–1391
Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zang TD, Waxman S, Chen Z, Chen GQ . Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations J Natl Cancer Inst 1999 91: 772–778
Gianni M, Koken M, Chelbi-Alix M, Benoit G, Lanotte M, Chen Z, de Thé H . Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells Blood 1998 91: 4300–4310
Dai J, Weinberg RS, Waxman S, Jing Y . Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathion redox system Blood 1999 93: 268–277
Akao Y, Nakagawa Y, Akiyama K . Arsenic trioxide induces apoptosis in neuroblastoma cell lines through the activation of caspase 3 in vitro FEBS Lett 1999 455: 59–62
Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G . Arsenic induces apoptosis via a direct effect on the mitochondrial permeability transition pore Exp Cell Res 1999 249: 413–421
Cai X, Shen YL, Zhu Q, Jia PM, Yu Y, Zhou L, Huang Y, Zhang JW, Xiong SM, Chen SJ, Wang ZY, Chen Z, Chen GQ . Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia Leukemia 2000 14: 262–270
Gross A, McDonnell JM, Korsmeyer JK . BCL-2 family members and the mitochondria in apoptosis Genes Dev 1999 13: 1899–1911
Los M, Wesselborg S, Schulze-Osthoff K . The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice Immunity 1999 10: 629–639
Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G . Mitochondrial permeability transition is a central coordinating event of apoptosis J Exp Med 1996 184: 1155–1160
Finucane DM, Waterhouse NJ, Amarante-Mendes GP, Cotter TG, Green DR . Collapse of the inner mitochondrial transmembrane potential is not required for apoptosis of HL60 cells Exp Cell Res 1999 251: 166–174
Lanotte M, Martin TV, Najman S, Balerini P, Valensi F, Berger R . NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3) Blood 1991 77: 1080–1086
Yoshida H, Kitamura K, Tanka K, Omura S, Miyazaki T, Hachiya T, Ohno R, Naoe T . Accelerated degradation of PML-RARα oncoprotein by all-trans retinoic acid in acute promyelocytic leukemia: possible role of the proteasome pathway Cancer Res 1996 56: 2945–2948
Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ . Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death J Biol Chem 1999 274: 1156–1163
Kitamura K, Kiyoi H, Yoshida H, Saito H, Ohno R, Naoe T . Mutant AF-2 domain of PML-RARα in retinoic acid-resistant NB4 cells: differentiation induced by RA is triggered directly through PML-RARα and its down-regulation in acute promyelocytic leukemia Leukemia 1997 11: 1950–1956
Naoe T, Kitamura K . Relationship between degradation of PML-RARalpha and differentiation Blood 1999 94: 1478–1479
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S . Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway Blood 1999 94: 2102–2111
Scott N, Hatlelid KM, MacKenzie NE, Carter DE . (1993) Reactions of arsenic (III) and arsenic (V) species with glutathione Chem Res Toxicol 1993 6: 102–106
Winski SL, Carter DE . Interactions of rat blood cell sulfhydryls with arsenate and arsenite J Toxicol Environ Health 1995 46: 379–397
Chen W, Martindale JL, Holbrook NJ, Liu Y . Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc Mol Cell Biol 1998 18: 5178–5188
Cavigelli M, Li WW, Lin A, Su B, Yoshida K, Karin M . The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase EMBO J 1996 15: 6269–6279
Porter AC, Fanger GR, Vaillancourt RR . Signal transduction pathways regulated by arsenate and arsenite Oncogene 1999 18: 7794–7802
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM . FADD, a novel deathdomain-containing protein, interacts with the death domain of Fas and initiates apoptosis Cell 1995 81: 505–512
Boldin MP, Goncharov TM, Goltsev YV, Wallach D . Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death Cell 1996 85: 803–815
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Lrammer PH, Peter ME, Dixit VM . FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex Cell 1996 85: 817–827
Kroemer G, Zamzami N, Susin SA . Mitochondrial control of apoptosis Immunol Today 1997 18: 44–51
Green DR, Reed JC . Mitochondria and apoptosis Science 1998 281: 1309–1312
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X . Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade Cell 1997 91: 479–489
Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, Kroemer G . The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis J Exp Med 1997 186: 25–37
Li H, Zhu H, Xu CJ, Yuan J . Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis Cell 1998 94: 491–501
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid, a Bcl2 interacting protein, mediates cytochrom c release from mitochondria in response to activation of cell surface death receptors Cell 1998 94: 481–490
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J . Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta Nature 2000 403: 98–103
Yang CH, Kuo ML, Chen JC, Chen YC . Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells Br J Cancer 1999 5: 796–799
Chen YC, Lin-Shiau SY, Lin JK . Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis J Cell Physiol 1998 177: 324–333
Eischen CM, Kottke TJ, Martins LM, Basi GS, Tung JS, Earnshaw WC, Leibson PJ, Kaufmann SH . Comparison of apoptosis in wild-type and Fas-resistant cells: chemotherapy-induced apoptosis is not dependent on Fas/Fas ligand interactions Blood 1997 90: 935–943
Boesen-de Cock JGR, de Vries E, Williams GT, Borst J . The anti-cancer drug etoposide can induce caspase-8 processing and apoptosis in the absence of CD95 receptor-ligand interaction Apoptosis 1998 3: 17–25
Micheau O, Solary E, Hammann A, Dimanche-Boitrel MT . Fas ligand-independent, FADD-mediated activation of the Fas death pathway by anticancer drugs J Biol Chem 1999 274: 7987–7992
Belka C, Marini P, Lepple-Wienhues A, Budach W, Jekle A, Los M, Lang F, Schulze-Osthoff K, Gulbins E, Bamberg M . The tyrosine kinase lck is required for CD95-independent caspase-8 activation and apoptosis in response to ionizing radiation Oncogene 1999 18: 4983–4992
Villunger A, Egle A, Kos M, Hartmann BL, Geley S, Kofler R, Greil R . Drug-induced apoptosis is associated with enhanced Fas (Apo-1/CD95) ligand expression but occurs independently of Fas (Apo-1/CD95) signaling in human T-acute lymphatic leukemia cells Cancer Res 1997 57: 3331–3334
Petak I, Tillman DM, Harwood FG, Mihalik R, Houghton JA . Fas-dependent and -independent mechanisms of cell death following DNA damage in human colon carcinoma cells Cancer Res 2000 60: 2643–2650
Fulda S, Strauss G, Meyer E, Debatin KM . Functional CD95 ligand and CD95 death-inducing signaling complex in activation-induced cell death and doxorubicin-induced apoptosis in leukemic T cells Blood 2000 95: 301–308
Iijima N, Miyamura K, Itou T, Tanimoto M, Sobue R, Saito H . Functional expression of Fas (CD95) in acute myeloid leukemia cells in the context of CD34 and CD38 expression: possible correlation with sensitivity to chemotherapy Blood 1997 90: 4901–4909
Acknowledgements
We are grateful to Dr SJ Korsmeyer for providing anti-Bid antibody. This work was partly supported by a Grant-in-Aid from the Japanese Ministry of Education and Culture.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitamura, K., Minami, Y., Yamamoto, K. et al. Involvement of CD95-independent caspase 8 activation in arsenic trioxide-induced apoptosis. Leukemia 14, 1743–1750 (2000). https://doi.org/10.1038/sj.leu.2401900
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401900
Keywords
This article is cited by
-
Clinical Indicators of Hepatotoxicity in Newly Diagnosed Acute Promyelocytic Leukemia Patients Undergoing Arsenic Trioxide Treatment
Biological Trace Element Research (2024)
-
Arsenic trioxide: applications, mechanisms of action, toxicity and rescue strategies to date
Archives of Pharmacal Research (2024)
-
Arsenic trioxide induces human pulmonary fibroblast cell death via the regulation of Bcl-2 family and caspase-8
Molecular Biology Reports (2012)
-
Tamoxifen enhances therapeutic effects of gemcitabine on cholangiocarcinoma tumorigenesis
Laboratory Investigation (2011)
-
Arsenic trioxide induces apoptosis preferentially in B-CLL cells of patients with unfavourable prognostic factors including del17p13
Journal of Molecular Medicine (2008)