Abstract
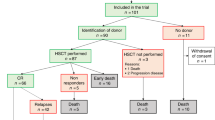
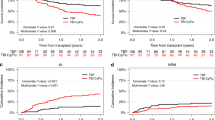
The purpose of this study was to assess the safety and efficacy of stem cell transplantation (SCT) mainly autologous SCT as consolidation therapy in APL patients who relapsed and achieved a second complete remission (CR2). Fifty adult patients with a first relapsed APL, of whom 39 had been previously treated with ATRA, entered a multicenter trial of oral ATRA until complete remission (CR) achievement followed by timed sequential chemotherapy (EMA combining etoposide 200 mg/m2/day for 3 days, mitoxantrone 12 mg/m2/day for 3 days, and cytarabine 500 mg/m2/day for two sequences of 3 days). ema was started either after cr achievement, or on day 1 of atra because of initial white blood cell (wbc) counts >5 × 109/l, or rapidly added to ATRA in order to prevent ATRA syndrome because WBC count increased under ATRA. Forty-five patients (90%, 95% CI 78%–97%) were in CR after induction therapy. Five patients died from infection during aplasia following EMA chemotherapy. Eleven patients who achieved CR had a familial HLA-identical donor and were allografted. The median disease-free survival (DFS) of allografted patients was 8.2 months. The 34 other CR patients were scheduled for autologous peripheral blood (PB) SCT (intent-to-treat group). Actually, autologous transplantation was only carried out in 22 patients (65%) (17 PBSCT and five autologous bone marrow transplantation (BMT)). Reasons for not autografting were early relapse (three patients), severe toxicity of EMA chemotherapy (six patients), and refusal or failure of stem cell harvest (three patients). The 3-year DFS rate of patients actually autografted was 77%. Among the 17 autografted patients still in CR2, nine patients have already reached a longer CR2 than first CR (CR1). Results of detection of PML/RARα by RT-PCR after autologous transplantation show negative findings in eight of the nine patients tested. We conclude that (1) ATRA combined to EMA chemotherapy is effective in the treatment of relapsed APL; (2) allogeneic BMT may be too toxic after salvage treatment including EMA intensive chemotherapy; (3) clinical outcome of autografted patients and preliminary molecular results regarding detection of PML/RARα after autologous PBSCT are encouraging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wang ZH . Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia Blood 1988 72: 567–572
Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L . All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results Blood 1990 76: 1704–1709
Fenaux P, Castaigne S, Dombret H, Archimbaud E, Duarte M, Morel P, Lamy T, Tilly H, Guerci A, Maloisel F, Bordessoule D, Sadoun A, Tiberghien P, Fegueux N, Daniel MT, Chomienne C, Degos L . All-trans retinoic acid followed by intensive chemotherapy gives a high complete remission rate and may prolong remissions in newly diagnosed acute promyelocytic leukemia: a pilot study on 26 cases Blood 1992 80: 2176–2181
Warrell RP, Frankel SR, Miller WH, Scheinberg DA, Itri LM, Hittelman WN, Vyas R, Andreeff M, Tafuri A, Jakubovski A, Gabrilove J, Gordon MS, Dmitrovsky E . Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans retinoic acid) New Engl J Med 1991 324: 1385–1393
Fenaux P, Le Deley MC, Castaigne S, Archimbaud E, Chomienne C, Link H, Guerci A, Duarte M, Daniel MT, Bowen D, Huebner G, Bauters F, Fegueux N, Fey M, Sanz M, Lowenberg B, Maloisel F, Auzanneau G, Sadoun A, Gardin C, Bastion Y, Ganser A, Jacky E, Dombret H, Chastang C, Degos L, and the European APL 91 Group . Effect of all-trans retinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial Blood 1993 82: 3241–3249
Thomas X, Anglaret B, Thiebaut A, Belhabri A, Treille-Ritouet D, Fiere D, Archimbaud E . Improvement of prognosis in refractory and relapsed acute promyelocytic leukemia over recent years: the role of all-trans retinoic acid therapy Ann Hematol 1997 75: 195–200
Cortes JE, Kantarjian H, O'Brien S, Robertson LE, Koller C, Hirsh-Ginsberg C, Stass S, Keating M, Estey E . All-trans retinoic acid followed by chemotherapy for salvage of refractory or relapsed acute promyelocytic leukemia Cancer 1994 73: 2946–2952
Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, Sale GE, Sanders JE, Singer JW, Shulman H, Storb R, Weiden PL . Marrow transplantation for acute nonlymphoblastic leukemia in first remission New Engl J Med 1979 301: 597–599
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, Gralnick HR . A variant form of hypergranular promyelocytic leukaemia (M3) Br J Haematol 1980 44: 169–170
Castaigne S, Balitrand N, De Thé H, Dejean A, Degos L, Chomienne C . A PML/retinoid acid receptor alpha fusion transcript is constantly detected by RNA-based polymerase chain reaction in acute promyelocytic leukemia Blood 1992 79: 3110–3115
Fenaux P, Chastang C, Chevret C, Sanz M, Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C, Cony Makhoul P, Travade P, Solary E, Fegueux N, Bordessoule D, San Miguel J, Link H, Desablens B, Stamatoullas A, Deconinck E, Maloisel F, Castaigne S, Preudhomme C, Degos L for the European APL group . A randomized comparison of all-trans retinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia Blood 1999 94: 1192–1200
Cordonnier C, Dreyfus F, Casassus P, Leblond V, Pesce A, Teilletthibault F, Colombat P, Troussard X, Dombret H, Gouault M, Jouault H, Kuentz M, Karianakis G, Vernant JP . Progressive induction for prevention of disseminated intravascular coagulation in acute promyelocytic leukaemia: preliminary results. In: Barbui T, Falanga A, Minetti B, Gorini S, Tognoni G, Donati MB (eds) Bergamo Spring Conferences on Haematology. Infections and Haemorrhage in Acute Leukaemia John Libbey Eurotext: Paris 1989 pp 81–87
Archimbaud E, Leblond V, Michallet M, Cordonnier C, Fenaux P, Travade P, Dreyfus D, Jaubert J, Devaux Y, Fiere D . Intensive sequential chemotherapy with mitoxantrone and continuous infusion etoposide and cytarabine for previously treated acute myelogenous leukemia Blood 1991 77: 1894–1900
Ellison RR, Holland JF, Weil M, Jacquillat C, Boiron M, Bernard J, Sawitsky A, Rosner F, Gussoff B, Silver RT, Karanas A, Cuttner J, Spurr CL, Hayes DM, Bloom J, Leone LA, Haurani F, Kyle R, Hutchison JL, Forcier RJ, Moon JH . Arabinosyl cytosine: a useful agent in the treatment of acute leukemia in adults Blood 1968 32: 507–523
Preisler HD . Failure of remission induction in acute myelocytic leukemia Med Pediatr Oncol 1978 4: 275–276
World Health Organization Handbook for Reporting Results of Cancer Treatment WHO Offset Publication: 48 WHO-Geneva 1979
Kaplan EL, Meier P . Non-parametric estimation from incomplete observations J Am Stat Assoc 1958 53: 457–481
Cox DR . Regression models and life tables JR Stat Soc B 1972 34: 187–220
Mantel N . Evaluation of survival data and two new rank order statistics arising in its consideration Cancer Chemother Rep 1966 50: 163–170
Gorin NC, Labopin M, Meloni G, Korbling M, Carella A, Herve P, Burnett A, Rizzoli V, Alessandrino EP, Bjorkstrand B, Ferrant A, Löwenberg B, Coser P, Simonsson B, Helbig W, Brunet Mauri S, Verdonck LF, Iriondi A, Polli E, Colombat P, Franklin IM, Souillet G, Willemze R . Autologous bone marrow transplantation for acute myeloid leukemia in Europe: further evidence of the role of marrow purging by mafosfamide. European Co-operative Group for Bone Marrow Transplantation (EBMT) Leukemia 1991 5: 896–904
Mandelli F, Labopin M, Granena A, Iriondo A, Prentice G, Bacigalupo A, Sierra J, Meloni G, Frassoni F, Goldman J, Gratwohl A, Gorin NC, and EBMT . European survey of bone marrow transplantation in acute promyelocytic leukemia (M3) Bone Marrow Transplant 1994 14: 293–298
Stein AS, O'Donell MR, Chai A, Schmidt GM, Nademanee A, Parker PM, Smith EP, Snyder DS, Molina A, Stepan DE, Spielberger R, Somlo G, Margolin KA, Vora N, Lipsett J, Lee J, Niland J, Forman SJ . In vivo purging with high-dose cytarabine followed by high-dose chemoradiotherapy and reinfusion of unpurged bone marrow for adult acute myelogenous leukemia in first complete remission J Clin Oncol 1996 14: 2206–2216
Diverio D, Pandolfi PP, Rossi V, Biondi A, Pelicci PG, Lo Coco F . Monitoring of treatment outcome in acute promyelocytic leukemia by RT-PCR Leukemia 1994 8: 1105–1107
Meloni G, Diverio D, Vignetti M, Avvisati G, Capria S, Petti MC, Mandelli F, Lo Coco F . Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pretransplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RARα fusion gene Blood 1997 90: 1321–1325
Roman J, Martin C, Torres A, Jimenez MA, Andres P, Flores R, de la Torre MJ, Sanchez J, Serrano J, Falcon M . Absence of detectable PML-RARα fusion transcripts in long-term remission patients after BMT for acute promyelocytic leukemia Bone Marrow Transplant 1997 19: 679–683
Castagnola C, Lunghi M, Corso A, Tajana M, Zappasodi P, Dabusti M, Lazzarino M, Bernasconi C . Management of acute promyelocytic leukemia relapse in the ATRA era Haematologica 1998 83: 714–717
Slack JL . Recent advances in the biology and treatment of acute promyelocytic leukemia Proc Am Soc Clin Oncol 1998 17: 54–65
Demirer T, Buckner CD, Appelbaum FR, Petersen FB, Rowley S, Weaver CH, Lilleby K, Sanders J, Chauncey T, Storb R, Schiffman K, Benyunes MC, Fefer A, Montgomery P, Bensinger WI . Rapid engraftment after autologous transplantation utilizing marrow and recombinant granulocyte colony-stimulating factor-mobilized peripheral blood stem cells in patients with acute myelogenous leukemia Bone Marrow Transplant 1995 15: 915–922
Gorin NC, Aegerter P, Auvert B, Meloni G, Goldstone AH, Burnett A, Carella A, Korbling M, Herve P, Maraninchi D, Löwenberg B, Verdonck LF, de Planque M, Hermans J, Helbig W, Porcellini A, Rizzoli V, Alesandrino EP, Franklin IM, Reiffers J, Colleselli P, Goldman JM . Autologous bone marrow transplantation for acute myelocytic leukemia in first remission: a European survey of the role of marrow purging Blood 1990 75: 1606–1614
Grimwade D . The pathogenesis of acute promyelocytic leukaemia: evaluation of the role of molecular diagnosis and monitoring in the management of the disease Br J Haematol 1999 106: 591–613
Sanz MA, de la Rubia J, Bonanad S, Barragan E, Sempere A, Martin G, Martinez JA, Jimenez C, Cervera J, Bolufer P, Sanz GF . Prolonged molecular remission after PML/RAR alpha-positive autologous peripheral blood stem cell transplantation in acute promyelocytic leukemia: is relevant pretransplant minimal residual disease in the graft? Leukemia 1998 12: 992–995
Carella AM, Cunningham I, Lerma E, Dejana A, Benvenuto F, Podesta M, Celesti L, Chimirri F, Abate M, Vassallo F, Figari O, Parodi C, Sessarego M, Valbonesi M, Carlier P, Prencipe E, Gatti AM, van den Berg D, Hoffman R, Frassoni F . Mobilization and transplantation of Philadelphia-negative peripheral-blood progenitors cells early in chronic myelogenous leukemia J Clin Oncol 1997 15: 1575–1582
Paietta E, Andersen J, Gallagher R, Bennett J, Yunis J, Cassileth P, Rowe J, Wiernik PH . The immunophenotype of acute promyelocytic leukemia (APL): an ECOG study Leukemia 1994 8: 1108–1112
Sierra J, Brunet S, Granena A, Olive T, Bueno J, Ribera JM, Petit J, Besses C, Llorente A, Guardia R, Macia J, Rovira M, Badell I, Vela E, Diaz de Heredia C, Vivancos P, Carreras E, Feliu E, Montserrat E, Julia A, Cubells J, Rozman C, Domingo A, Ortega JJ . Feasibility and results of bone marrow transplantation after remission induction and intensification chemotherapy in de novo acute myeloid leukemia. Catalan Group for Bone Marrow Transplantation J Clin Oncol 1996 14: 1353–1363
Jourdan E, Maraninchi D, Reiffers J, Gluckman E, Rio B, Jouet JP, Michallet M, Molina L, Archimbaud E, Harousseau JL, Ifrah N, Attal M, Guilhot F, Kuentz M, Guyotat D, Pico JL, Dauriac C, Legros M, Dreyfus F, Bordigoni P, Leblond V, Gratecos N, Varet B, Auzaneau C, Tilly H, Vilmer E, Bardou VJ, Blaise D for the Société Française de Greffe de Moelle (SFGM) . Early allogeneic transplantation favorably influences the outcome of adult patients suffering from acute myeloid leukemia Bone Marrow Transplant 1997 19: 875–881
Miller WH Jr, Kakizuka A, Frankel SR, Warrell RP Jr, De Blasio A, Levine K, Evans RM, Dmitrovsky E . Reverse transcription polymerase chain reaction for the rearranged retinoic acid receptor α clarifies diagnosis and detects minimal residual disease in acute promyelocytic leukemia Proc Natl Acad Sci USA 1992 89: 2694–2698
Lo Coco F, Diverio D, Pandolfi PP, Biondi A, Rossi V, Avvisati G, Rambaldi A, Arcese W, Petti MC, Meloni G, Mandelli F, Grignani F, Masera G, Barbui T, Pelicci PG . Molecular evaluation of residual disease as a predictor of relapse in acute promyelocytic leukemia Lancet 1992 340: 1437–1438
Huang W, Sun GL, Li XS, Cao Q, Lu Y, Jang GS, Zhang FQ, Chai JR, Wang ZY, Waxman S, Chen Z, Chen SJ . Acute promyelocytic leukemia: relevance of two major PML-RARα isoforms and detection of minimal residual disease by retrotransferase-polymerase chain reaction Blood 1993 82: 1264–1269
Grimwade D, Jamal R, Goulden N, Kempski H, Mastrangelo S, Veys P . Salvage of patients with acute promyelocytic leukaemia with residual disease following ABMT performed in second CR using all-trans retinoic acid Br J Haematol 1998 103: 559–562
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr . Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide New Engl J Med 1998 339: 1341–1348
Lo Coco F, Diverio D, Avvisati G, Petti MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C, Selleri C, Martino B, Specchia G, Mandelli F . Therapy of molecular relapse in acute promyelocytic leukemia Blood 1999 94: 2225–2229
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY . Use of arsenic trioxide (AS2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients Blood 1997 89: 3354–3360
Acknowledgements
This work was supported by PHRC 1995 (CHU Lille).
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Thomas, X., Dombret, H., Cordonnier, C. et al. Treatment of relapsing acute promyelocytic leukemia by all-trans retinoic acid therapy followed by timed sequential chemotherapy and stem cell transplantation. Leukemia 14, 1006–1013 (2000). https://doi.org/10.1038/sj.leu.2401800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401800
Keywords
This article is cited by
-
Acute Promyelocytic Leukemia: A History over 60 Years—From the Most Malignant to the most Curable Form of Acute Leukemia
Oncology and Therapy (2019)
-
Arsenic trioxide-based therapy of relapsed acute promyelocytic leukemia: registry results from the European LeukemiaNet
Leukemia (2015)
-
Progress in the treatment of acute promyelocytic leukemia: optimization and obstruction
International Journal of Hematology (2014)
-
Management of elderly patients with acute promyelocytic leukemia: progress and problems
Annals of Hematology (2013)