Abstract
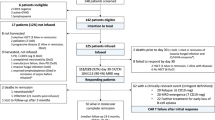
Treatment of children with acute lymphoblastic leukaemia (ALL) aims to cure all patients with as little toxicity as possible and, if possible, to restrict further intensification of chemotherapy to patients with an increased risk of relapse. However in Medical Research Council (MRC) trial UKALL X two short myeloablative blocks of intensification therapy given at weeks 5 and 20 were of benefit to children in all risk groups. The successor trials, MRC UKALL XI and MRC ALL97, tested whether further intensification would continue to benefit all patients by randomising them to receive, or not, an extended third intensification block at week 35. After a median follow-up of 4 years (range 5 months to 8 years), 5 year projected event-free survival was superior at 68% for the 894 patients allocated a third intensification compared with 60% for the 887 patients who did not receive one (odds ratio 0.75, 95% CI 0.63–0.90, 2P = 0.002). This difference was almost entirely due to a reduced incidence of bone marrow relapses in the third intensification arm (140 of 891 in the third intensification arm vs 171 of 883 in the no third intensification, 2P = 0.02). Subgroup analysis suggests benefit of the third intensification for all risk categories. Overall survival to date is no different in the two arms, indicating that a greater proportion of those not receiving a third intensification arm and subsequently relapsing can be salvaged. These results indicate that there is benefit of additional intensification for all risk subgroups of childhood ALL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rubnitz JE, Look AT . Molecular genetics of childhood leukemias J Pediatr Hematol Oncol 1998 20: 1–11
Chessells JM, Swansbury GJ, Reeves B, Bailey CC, Richards SM . Cytogenetics and prognosis in childhood lymphoblastic leukaemia: results of MRC UKALL X. Medical Research Council Working Party in Childhood Leukaemia Br J Haematol 1997 99: 93–100
Brisco MJ, Condon J, Hughes E, Neoh SH, Sykes PJ, Seshadri R, Toogood I, Waters K, Tauro G, Ekert H . Outcome prediction in childhood acute lymphoblastic leukaemia by molecular quantification of residual disease at the end of induction Lancet 1994 343: 196–200
Cave H, van der Werff ten Bosch, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E . Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer–Childhood Leukemia Cooperative Group New Engl J Med 1998 339: 591–598
van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, Stolz F, Schrappe M, Masera G, Kamps WA, Gadner H, van Wering ER, Ludwig WD, Basso G, de Bruijn MA, Cazzaniga G, Hettinger K, van der Does-van den Berg A, Hop WC, Riehm H, Bartram CR . Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood Lancet 1998 352: 1731–1738
Ochs J, Mulhern R . Long-term sequelae of therapy for childhood acute lymphoblastic leukaemia Baillières Clin Haematol 1994 7: 365–376
Chessells JM, Richards SM, Bailey CC, Lilleyman JS, Eden OB . Gender and treatment outcome in childhood lymphoblastic leukaemia: report from the MRC UKALL trials Br J Haematol 1995 89: 364–372
Chessells JM, Hall E, Prentice HG, Durrant J, Bailey CC, Richards SM . The impact of age on outcome in lymphoblastic leukaemia; MRC UKALL X and XA compared: a report from the MRC Paediatric and Adult Working Parties Leukemia 1998 12: 463–473
Pui CH . Recent advances in the biology and treatment of childhood acute lymphoblastic leukemia Curr Opin Hematol 1998 5: 292–301
Hutchinson RJ, Neerhout S, Bertolone S, Cooper HA, Roskos R, Tannous R, Wells L, Heerema N, Hummell D, Sailoer S, Sather H, Trigg M and the Children's Cancer Study Group . Should therapy be intensified for patients with good risk acute lymphoblastic leukaemia Am Soc Hematol 1996 A2662
Rivera GK, Pinkel D, Simone JV, Hancock ML, Crist WM . Treatment of acute lymphoblastic leukemia. 30 years’ experience at St Jude Children's Research Hospital New Engl J Med 1993 329: 1289–1295
Tubergen DG, Gilchrist GS, O'Brien RT, Coccia PF, Sather HN, Waskerwitz MJ, Hammond GD . Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial J Clin Oncol 1993 11: 520–526
Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, Odenwald E, Ritter J, Mann G, Welte K, Gadner H, Riehm H . Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86 Blood 1994 84: 3122–3133
Chessells JM, Bailey C, Richards SM . Intensification of treatment and survival in all children with lymphoblastic leukaemia: results of UK Medical Research Council trial UKALL X. Medical Research Council Working Party on Childhood Leukaemia Lancet 1995 345: 143–148
Hill F, Hann I, Gibson B, Eden OB, Richards S . Comparison of high dose methotrexate with continuing intrathecal methotrexate versus intrathecal methotrexate alone in low white blood count childhood acute lymphoblastic leukaemia: preliminary results from the UKALLXI randomised trial Blood 1998 92: (Suppl. 1) 398a
White SJ, Freedman LS . Allocation of patients to treatment groups in a controlled clinical study Br J Cancer 1978 37: 849–857
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG . Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II Analysis and examples Br J Cancer 1977 35: 1–39
Early Breast Cancer Trialists’ Collaborative Group . Systemic treatment of early breast cancer by hormonal cytotoxic, or immune therapy. 133 randomised trials involving 31 000 recurrences and 24000 deaths among 75000 women Lancet 1992 339: 1–15
Cox D . The contribution of statistical methods to cancer research Cancer 1991 67: 2428–2430
SAS/STAT User's Guide SAS Institute Inc: Cary, NC 1989
Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, Johnstone HS, Sather HN Trigg ME, Uckun FM, Bleyer WA . Treatment of patients with acute lymphoblastic leukemia with bulky extra-medullary disease and T-cell phenotype or other poor prognostic features: randomized controlled trial from the Children's Cancer Group Cancer 1998 82: 600–612
Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS . Augmented post-induction therapy for children with high-risk acute lymphoblastic leukaemia and a slow response to initial therapy New Engl J Med 1998 338: 1663–1671
Lange B, Sather H, Weetman R, Bostrom B, McGuire P, Rackoff W, Arthur D, Trigg M for the Children's Cancer Group . Double delayed intensification improves outcome in moderate risk paediatric acute lymphoblastic leukaemia – CCG 1891 study ASH 1997 90: Suppl. 1: 559a
Henze G, Fengler R, Reiter A, Ritter J, Riehm H . Impact of early intensive reinduction therapy on event-free survival in children with low-risk acute lymphoblastic leukemia Hamatol Bluttransfus 1990 33: 483–488
Morley A . Quantifying leukemia Lancet 1998 339: 627–629
Acknowledgements
We are grateful to all the physicians who entered patients to these studies. Members of the MRC childhood leukaemia working party during these studies were: Bailey CC, Barton C, Broadbent V, Caswell M, Chessells JM, Darbyshire PJ, Dempsey SI, Durrant J, Eden OB (Chairman), Gibson B, Goodman A, Gray R, Hann I, Haworth C, Forman K, Hill F, Jenney M, Kernahan J, King DJ, Kinsey SE, Madden M, Mann JR, Martin J, Meller ST, Mitchell C, Oakhill A, Radford M, Reid MM, Richards SM, Smyth O, Stevens RF, Thomas A, Vargha-Khadem F, Vora AJ, Walker D, Webb D, Wheatley K, Will A, Windebank K. We also thank J Burrett for data management and all those who worked in the randomisation office.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Hann, I., Vora, A., Richards, S. et al. Benefit of intensified treatment for all children with acute lymphoblastic leukaemia: results from MRC UKALL XI and MRC ALL97 randomised trials. Leukemia 14, 356–363 (2000). https://doi.org/10.1038/sj.leu.2401704
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401704
Keywords
This article is cited by
-
NT5E gene and CD38 protein as potential prognostic biomarkers for childhood B-acute lymphoblastic leukemia
Purinergic Signalling (2022)
-
Antioxidant Levels at Diagnosis in Childhood Acute Lymphoblastic Leukemia
The Indian Journal of Pediatrics (2013)
-
Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations
Leukemia (2012)
-
Long-term follow-up of the United Kingdom medical research council protocols for childhood acute lymphoblastic leukaemia, 1980–2001
Leukemia (2010)
-
Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985–2001
Leukemia (2010)