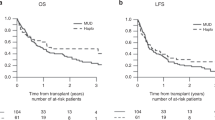
Abstract
The number of allogeneic transplants from unrelated donors has grown in the past decade in part because of the expansion of the donor registry size. Patient survival has improved due to the selection of more closely matched donors and the development of effective infection prophylaxis. Relapse-free survival remains limited in patients with a large tumor burden at the time of transplantation. A higher marrow cell dose is the major factor to minimize transplant-related death. Future studies of peripheral blood stem cell transplants should be considered for patients with acute leukemia with the goal of enhancing the stem cell dose and improving survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Horowitz MM, Rowlings PA . An update from the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry on current activity in hematopoietic stem cell transplantation Curr Opin Hematol 1997 4: 395–400
Anonymous . Bone Marrow Donors Worldwide Internet address: http://www.bmdw.org
Sierra J, Storer B, Hansen JA, Bjerke JW, Martin PJ, Petersdorf EW, Appelbaum FR, Bryant E, Chauncey TR, Sale G, Sanders JE, Storb R, Sullivan KM, Anasetti C . Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose Blood 1997 89: 4226–4235
Ringden O, Remberger M, Runde V, Bornhauser M, Blau IW, Basara N, Holig K, Beelen DW, Hagglund H, Basu O, Ehninger G, Fauser AA . Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation Blood 1999 94: 455–464
Gluckman E, Rocha V, Boyer-Chammard A, Locatelli F, Arcese W, Pasquini R, Ortega J, Souillet G, Ferreira E, Laporte JP, Fernandez M, Chastang C . Outcome of cord-blood transplantation from related and unrelated donors New Engl J Med 1997 337: 373–381
Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE . Outcomes among 562 recipients ofplacental-blood transplants from unrelated donors New Engl J Med 1998 339: 1565–1577
Mavroudis D, Read E, Cottler-Fox M, Couriel D, Molldrem J, Carter C, Yu M, Dunbar C, Barrett J . CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies Blood 1996 88: 3223–3229
Wagner JE, King R, Kollman K, Anasetti C, Antin J, Casper J, Champlin R, Davies SM, Drobyski W, Fay J, Filipovich A, Heslop H, Karanes C, Kernan N, Nademanee A, Schmeckpeper B, Howe C . Unrelated donor bone marrow transplantation in 5075 patients with malignant and nonmalignant disorders: impact of marrow T cell depletion Blood 1998 92: 686a (Abstr.)
Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, Gillio AP, Kernan NA, Small TN, Szabolcs P, Taylor J, Yahalom J, Collins NH, Bleau SA, Black PM, Heller G, O'Reilly RJ, Young JW . T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease Blood 1998 91: 1083–1090
Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF . Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with on fully mismatched HLA haplotype New Engl J Med 1998 339: 1186–1193
Hansen JA, Gooley TA, Martin PJ, Appelbaum F, Chauncey TR, Clift RA, Petersdorf EW, Radich J, Sanders JE, Storb RF, Sullivan KM, Anasetti C . Bone marrow transplants from unrelated donors for patients with chronic myeloid leukemia New Engl J Med 1998 338: 962–968
Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM, Woolfrey AE, Hansen JA . Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient Blood 1998 92: 3515–3520
Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoki H, Yoskida T, Kimura A, Akaza T, Kamikawaji N, Kodera Y, Takaku R . Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program New Engl J Med 1998 339: 1177–1185
Anasetti C, Heimfeld S, Rowley S, Martin P, Petersdorf EW, Woolfrey A, Hansen JA . Higher CD34 cell dose is associated with improved survival after marrow transplantation from unrelated donors Blood 1999 94: (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anasetti, C. Transplantation of hematopoietic stem cells from alternate donors in acute myelogenous leukemia. Leukemia 14, 502–504 (2000). https://doi.org/10.1038/sj.leu.2401648
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2401648
Keywords
This article is cited by
-
Current status of haploidentical stem cell transplantation for leukemia
Journal of Hematology & Oncology (2008)
-
Haploidentical hematopoietic cell transplantation
Bone Marrow Transplantation (2008)
-
Genetically haploidentical stem cell transplantation for acute leukemia
Bone Marrow Transplantation (2001)