Abstract
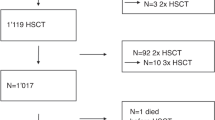
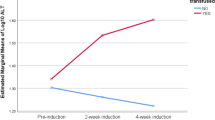
Allogeneic hematopoietic stem cell transplantation (ASCT) and its conditioning with chemoradiotherapy often results in liver toxicity, the most severe form being veno-occlusive liver disease (VOD). N-acetyl-L-cysteine (NAC), an antioxidant glutathione precursor, may provide protection from liver toxicity. Patients with elevated bilirubin (>26 mmol/l) and/or elevated (ALT) (>1.4 μkat/l) and/or aspartate aminotransferase (AST) (>1.4 μkat/l) levels were randomized to treatment with NAC or no treatment. Among 522 transplanted patients, 160 were included in the trial. NAC was given, 100 mg/kg per day, as a 6-h i.v. infusion until normalization of bilirubin, ALT and AST values. Maximum bilirubin level was the same in patients randomized to NAC (n=72) or controls (n=88). Increase and recovery of ALT and AST were the same in patients randomized to NAC or controls. There were two patients in the NAC group who developed VOD, as compared to three of the controls. To conclude, NAC does not improve liver toxicity after ASCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chyka PA, Butler AY, Holliman BJ, Herman MI . Utility of acetylcysteine in treating poisonings and adverse drug reactions. Drug Saf 2000; 22: 123–148.
Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R . Improved outcome of paracetamol-induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 1990; 335: 1572–1573.
Bonanomi L, Gazzaniga A . Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur J Respir Dis Suppl 1980; 111: 45–51.
Sekharam M, Trotti A, Cunnick JM, Wu J . Suppression of fibroblast cell cycle progression in G1 phase by N-acetylcysteine. Toxicol Appl Pharmacol 1998; 149: 210–216.
Colombo A, Ranheim E, Fong T, Kipps T . CD40 signaling in B cells involves reactive oxygen intermediates and induces activation of NF-kB. Blood 1994; 84 (Suppl 1): 511.
Ringden O, Remberger M, Lehmann S, Hentschke P, Mattsson J, Klaesson S et al. N-acetylcysteine for hepatic veno-occlusive disease after allogeneic stem cell transplantation. Bone Marrow Transplant 2000; 25: 993–996.
Sjoo F, Aschan J, Barkholt L, Hassan Z, Ringden O, Hassan M . N-acetyl-L-cysteine does not affect the pharmacokinetics or myelosuppressive effect of busulfan during conditioning prior to allogeneic stem cell transplantation. Bone Marrow Transplant 2003; 32: 349–354.
Ruutu T, Eriksson B, Remes K, Juvonen E, Volin L, Remberger M et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 2002; 100: 1977–1983.
Carreras E . Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol 2000; 64: 281–291.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
Shulman HM, Hinterberger W . Hepatic veno-occlusive disease—liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant 1992; 10: 197–214.
Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood 1990; 75: 1011–1016.
Remberger M, Ringden O . Increased levels of soluble interleukin-2 receptor in veno-occlusive disease of the liver after allogenic bone marrow transplantation. Transplantation 1995; 60: 1293–1299.
Remberger M, Ringden O . Serum levels of cytokines after bone marrow transplantation: increased IL-8 levels during severe veno-occlusive disease of the liver. Eur J Haematol 1997; 59: 254–262.
Shulman HM, Gown AM, Nugent DJ . Hepatic veno-occlusive disease after bone marrow transplantation. Immunohistochemical identification of the material within occluded central venules. Am J Pathol 1987; 127: 549–558.
Schaffer M, Aldener-Cannava A, Remberger M, Ringden O, Olerup O . Roles of HLA-B, HLA-C and HLA-DPA1 incompatibilities in the outcome of unrelated stem-cell transplantation. Tissue Antigens 2003; 62: 243–250.
Ringden O, Remberger M, Runde V, Bornhauser M, Blau IW, Basara N et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood 1999; 94: 455–464.
Ringden O, Remberger M, Svahn BM, Barkholt L, Mattsson J, Aschan J et al. Allogeneic hematopoietic stem cell transplantation for inherited disorders: experience in a single center. Transplantation 2006; 81: 718–725.
Ringden O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindelov L et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood 1994; 83: 2723–2730.
Hentschke P, Barkholt L, Uzunel M, Mattsson J, Wersall P, Pisa P et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant 2003; 31: 253–261.
Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood 2003; 101: 1620–1629.
Remberger M, Svahn BM, Hentschke P, Lofgren C, Ringden O . Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant 1999; 24: 823–830.
Ringden O, Remberger M, Persson U, Ljungman P, Aldener A, Andstrom E et al. Similar incidence of graft-versus-host disease using HLA-A, -B and -DR identical unrelated bone marrow donors as with HLA-identical siblings. Bone Marrow Transplant 1995; 15: 619–625.
Storb R, Deeg HJ, Fisher L, Appelbaum F, Buckner CD, Bensinger W et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood 1988; 71: 293–298.
Svahn BM, Remberger M, Myrback KE, Holmberg K, Eriksson B, Hentschke P et al. Home care during the pancytopenic phase after allogeneic hematopoietic stem cell transplantation is advantageous compared with hospital care. Blood 2002; 100: 4317–4324.
Remberger M, Naseh N, Aschan J, Barkholt L, LeBlanc K, Svennberg P et al. G-CSF given after haematopoietic stem cell transplantation using HLA-identical sibling donors is associated to a higher incidence of acute GVHD II–IV. Bone Marrow Transplant 2003; 32: 217–223.
Lundgren G, Wilczek H, Lonnqvist B, Lindholm A, Wahren B, Ringden O . Acyclovir prophylaxis in bone marrow transplant recipients. Scand J Infect Dis Suppl 1985; 47: 137–144.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Tolar J, Orchard PJ, Bjoraker KJ, Ziegler RS, Shapiro EG, Charnas L . N-acetyl-L-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transplant 2007; 39: 211–215.
Wang X, Kanel GC, DeLeve LD . Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology 2000; 31: 428–434.
Hagglund H, Remberger M, Klaesson S, Lonnqvist B, Ljungman P, Ringden O . Norethisterone treatment, a major risk-factor for veno-occlusive disease in the liver after allogeneic bone marrow transplantation. Blood 1998; 92: 4568–4572.
Hassan Z, Ljungman P, Ringden O, Winiarski J, Nilsson C, Aschan J et al. Pharmacokinetics of liposomal busulphan in man. Bone Marrow Transplant 2001; 27: 479–485.
Ringden O, Barrett AJ, Zhang MJ, Loberiza FR, Bolwell BJ, Cairo MS et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol 2003; 121: 874–885.
Paulin T, Ringden O, Lonnqvist B . Faster immunological recovery after bone marrow transplantation in patients without cytomegalovirus infection. Transplantation 1985; 39: 377–384.
Paulin T, Ringden O, Nilsson B, Lonnqvist B, Gahrton G . Variables predicting bacterial and fungal infections after allogeneic marrow engraftment. Transplantation 1987; 43: 393–398.
Eapen M, Horowitz MM, Klein JP, Champlin RE, Loberiza Jr FR, Ringden O et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol 2004; 22: 4872–4880.
Acknowledgements
We thank the Staff and Centre for Allogeneic Stem Cell Transplantation for compassionate and competent care of the patients. We thank Inger Hammarberg for secretarial help. This study was supported by grants from the Swedish Cancer Society (0070-B06-20XBC), the Children's Cancer Foundation (06/094), the Swedish Research Council (K2006-32X-05971-26-1), the Cancer Society in Stockholm, the Cancer and Allergy Foundation and Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkholt, L., Remberger, M., Hassan, Z. et al. A prospective randomized study using N-acetyl-L-cysteine for early liver toxicity after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 41, 785–790 (2008). https://doi.org/10.1038/sj.bmt.1705969
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705969
Keywords
This article is cited by
-
Real-world use of defibrotide for veno-occlusive disease/sinusoidal obstruction syndrome: the DEFIFrance Registry Study
Bone Marrow Transplantation (2023)
-
A stitch in time saves nine: timely use of N-acetyl cysteine (NAC) for chemotherapy-induced veno-occlusive disease (VOD)—is it a cost-effective alternative?
Supportive Care in Cancer (2022)
-
The effect of N-acetyl-l-cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation
Scientific Reports (2018)
-
N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trial
Bone Marrow Transplantation (2014)
-
Incidence, characteristics and risk factors of marked hyperbilirubinemia after allogeneic hematopoietic cell transplantation with reduced-intensity conditioning
Bone Marrow Transplantation (2012)