Abstract
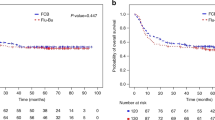
The current study aimed to evaluate the efficacy and toxicity of a combination of intravenous busulfan, cyclophosphamide and etoposide (i.v. Bu/Cy/E) as a conditioning regimen prior to autologous hematopoietic stem cell transplantation in patients with non-Hodgkin's lymphoma (NHL). Sixty-four patients with relapsed/refractory (n=36) or high-risk (n=28) lymphoma were enrolled. The high-dose chemotherapy consisted of i.v. Bu (0.8 mg kg−1 i.v. q 6 h from day −7 to day −5), Cy (50 mg kg−1 i.v. on day −3 and day −2) and E (400 mg m−2 i.v. on day −5 and day −4). The median age was 43 (range 18–65) years, and 39 patients were male. Diffuse large B-cell lymphoma (40.6%) was the most common histological subtype. All evaluable patients achieved an engraftment of neutrophils (median, day 12) and platelets (median, day 13). Hepatic veno-occlusive disease was observed in four patients (three mild, one moderate grade), and two patients (3.1%) died from treatment-related complications. At a median follow-up of 16.4 months, 15 patients (23.4%) exhibited a relapse or progression, while 13 patients (20.3%) had died of disease. The estimated 3-year overall and progression-free survival for all patients was 72.1 and 70.1%, respectively. In conclusion, the conditioning regimen of i.v. Bu/Cy/E was well tolerated and seemed to be effective in patients with aggressive NHL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Horning SJ, Armitage JO . Autologous hemotopoietic cell transplantation for non-Hodgkin's lymphoma. In: Blume K, Forman S, Appelbaum FR (eds). Thomas' Hematopoietic Cell Transplantation, 3rd edn. Blackwell Publishing: Malden, MA, 2004, pp 1207–1220.
Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin's lymphoma. N Engl J Med 1987; 316: 1493–1498.
Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–1545.
Takvorian T, Canellos GP, Ritz J, Freedman AS, Anderson KC, Mauch P et al. Prolonged disease-free survival after autologous bone marrow transplantation in patients with non-Hodgkin's lymphoma with a poor prognosis. N Engl J Med 1987; 316: 1499–1505.
Gulati S, Yahalom J, Acaba L, Reich L, Motzer R, Crown J et al. Treatment of patients with relapsed and resistant non-Hodgkin's lymphoma using total body irradiation, etoposide, and cyclophosphamide and autologous bone marrow transplantation. J Clin Oncol 1992; 10: 936–941.
Vose JM, Armitage JO, Bierman PJ, Weisenburger DD, Hutchins M, Dowling MD et al. Salvage therapy for relapsed or refractory non-Hodgkin's lymphoma utilizing bone marrow transplantation. Am J Med 1989; 87: 285–288.
Pettengell R, Radford JA, Morgenstern GR, Scarffe JH, Harris M, Woll PJ et al. Survival benefit from high-dose therapy with autologous blood progenitor-cell transplantation in poor-prognosis non-Hodgkin's lymphoma. J Clin Oncol 1996; 14: 586–592.
Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med 2004; 350: 1287–1295.
Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Copelan EA, Penza SL, Pohlman B, Avalos BR, Goormastic M, Andresen SW et al. Autotransplantation following busulfan, etoposide and cyclophosphamide in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 2000; 25: 1243–1248.
Hanel M, Kroger N, Sonnenberg S, Bornhauser M, Kruger W, Kroschinsky F et al. Busulfan, cyclophosphamide, and etoposide as high-dose conditioning regimen in patients with malignant lymphoma. Ann Hematol 2002; 81: 96–102.
Kroger N, Hoffknecht M, Hanel M, Kruger W, Zeller W, Stockschlader M et al. Busulfan, cyclophosphamide and etoposide as high-dose conditioning therapy in patients with malignant lymphoma and prior dose-limiting radiation therapy. Bone Marrow Transplant 1998; 21: 1171–1175.
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 2002; 8: 477–485.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 1995; 16: 31–42.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 2002; 8: 145–154.
Kashyap A, Wingard J, Cagnoni PJ, Roy J, Tarantolo S, Hu W et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 2002; 8: 493–500.
Olavarria E, Hassan M, Eades A, Nilsson C, Timms A, Matthews J et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. Leukemia 2000; 14: 1954–1959.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 1244–1253.
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Veno-occlusive disease of the liver after bone marrow transplantation. Hepatology 1984; 4: 116–122.
Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the bone marrow transplant survivor study. Blood 2005; 105: 4215–4222.
Avalos BR, Klein JL, Kapoor PJ, Tutschka PJ, Klein JP, Copelan FA . Preparation for marrow transplantation in Hodgkin's and non-Hodgkin's lymphoma using Bu/CY. Bone Marrow Transplant 1993; 12: 133–138.
Copelan EA, Bechtel TP, Avalos BR, Elder PJ, Ezzone SA, Scholl MD et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complication following marrow transplantation. Bone Marrow Transplant 2001; 27: 1121–1124.
Dix SP, Wingard JR, Brundrett RB, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Aggarwal C, Gupta S, Vaughan W, Saylors GB, Salzman DE, Katz RO et al. Improved outcomes in intermediate- and high-risk aggressive non-Hodgkin's lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral busulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biol Blood Marrow Transplant 2006; 12: 770–777.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Sohn, S., Chae, Y. et al. Multicenter study of intravenous busulfan, cyclophosphamide, and etoposide (i.v. Bu/Cy/E) as conditioning regimen for autologous stem cell transplantation in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant 40, 919–924 (2007). https://doi.org/10.1038/sj.bmt.1705841
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705841
Keywords
This article is cited by
-
A gemcitabine-based regimen followed by autologous stem cell transplantation show high efficacy and well tolerance in malignant lymphoma
Bone Marrow Transplantation (2022)
-
Phase II study of safety and efficacy of BEB (bendamustine, etoposide, and busulfan) conditioning regimen for autologous stem cell transplantation in non-Hodgkin lymphoma
Annals of Hematology (2020)
-
Strategies to improve outcomes of autologous hematopoietic cell transplant in lymphoma
Bone Marrow Transplantation (2019)
-
BEAM or BUCYVP16-conditioning regimen for autologous stem-cell transplantation in non-Hodgkin’s lymphomas
Bone Marrow Transplantation (2019)
-
Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: a retrospective study from the EBMT
Bone Marrow Transplantation (2016)