Abstract
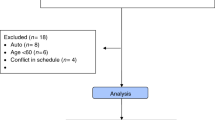
The aim of the study was to assess cognitive performance in patients with hematological malignancies before, and 3 months after, allogeneic hematopoietic stem cell transplant (HSCT). A consecutive sample of 39 patients was assessed before admission with a comprehensive neuropsychological test battery and health-related quality-of-life (HRQoL) questionnaires; 19 of these patients were retested around 100 days post HSCT. Test results were compared with normative data and revealed minimal differences at both time points in the level of group-means. One parameter – simple reaction time – was significantly worse (prolonged) at second measurement after HSCT. According to the definition of an impairment score (more than three impaired functions), 26% of patients were classified as impaired before as well as after HSCT. Neuropsychological test results did not vary systematically according to medical variables such as extent of pretreatment, graft-versus-host-disease (GvHD) and kind of conditioning protocol. As a dimension of HRQoL, self-rated cognitive function was in the normal range before and after HSCT. Significant correlations between HRQoL and neuropsychological parameters were related to symptom scales. This study showed impairments of neuropsychological performance for a subgroup of patients before and after allogeneic HSCT. Systematic effects of conditioning, medical variables or self-rated HRQoL could not be observed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ahles T, Tope D, Furstenberg C, Herndon J, Maurer LH, Cornblith AB et al. Psychologic and neuropsychologic impact of autologous bone marrow transplantation. J Clin Oncol 1996; 14: 1457–1462.
Meyers CA, Weitzner M, Byrne K, Valentine A, Champlin RE, Przepiorka D . Evaluation of the neurobehavioral functioning of patients before, during, and after bone marrow transplantation. J Clin Oncol 1994; 12: 820–826.
Wefel JS, Kayl AE, Meyers CA . Neuropsychological dysfunction associated with cancer and cancer therapies: a conceptual review of an emerging target. Br J Cancer 2004; 90: 1691–1696.
Andrykowski MA, Altmaier EM, Barnett RL, Burish TG, Gingrich R, Henslee-Downey PJ . Cognitive dysfunction in adult survivors of allogeneic marrow transplantation: relationship to dose of total body irradiation. Bone Marrow Transplant 1990; 6: 269–276.
Hjermstad MJ, Evensen SA, Kvaloy SO, Fayers PM, Kaasa S . Health-related quality of life 1 year after allogeneic or autologous stem-cell transplantation: a prospective study. J Clin Oncol 1999; 17: 706–718.
Andrykowski MA, Schmitt FA, Gregg ME, Brady MJ, Lamb DG, Henslee-Downey PJ . Neuropsychologic impairment in adult bone marrow transplant candidates. Cancer 1992; 70: 2288–2297.
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA . ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer 2004; 101: 466–475.
Harder H, Van Gool AR, Cornelissen JJ, Duivenvoorden HJ, Eijkenboom WM, Barge RM et al. Assessment of pre-treatment cognitive performance in adult bone marrow or haematopoietic stem cell transplantation patients: a comparative study. Eur J Cancer 2005; 41: 1007–1016.
Harder H, Cornelissen JJ, Van Gool AR, Duivenvoorden HJ, Eijkenboom WM, van den Bent MJ . Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer 2002; 95: 183–192.
Padovan CS, Yousry TA, Schleuning M, Holler E, Kolb HJ, Straube A . Neurological and neuroradiological findings in long-term survivors of allogeneic bone marrow transplantation. Ann Neurol 1998; 43: 627–633.
Sostak P, Padovan CS, Yousry TA, Ledderose G, Kolb HJ, Straube A . Prospective evaluation of neurological complications after allogeneic bone marrow transplantation. Neurology 2003; 60: 842–848.
Syrjala KL, Dikmen S, Langer S, Ruth-Roemer S, Abrams JR . Neuropsychological changes from pretransplant to one year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood 2004; 104: 3386–3392.
Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE . Neuropsychological effects of treatment for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc 2003; 9: 967–982.
Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J . A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer 2005; 104: 2222–2233.
Reitan RM . Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory: Tucson, AZ, 1979.
Zimmermann P, Fimm B . TAP Testbatterie zur Aufmerksamkeitsprüfung, Version 1.7. Psytest: Herzogenrath, 2002.
Brickenkamp R . Test d2 Aufmerksamkeits-Belastungs-Test, 9. Auflage. Hogrefe: Göttingen, 2002.
Wechsler D . WMS-R: Wechsler Memory Scale – Revised (Manual). The Psychological Corporation: San Antonio, 1987.
Härting C, Markowitsch HJ, Neufeld H, Calabrese P, Deisinger K (Hrsg.). WMS-R Wechsler Gedächtnistest – revidierte Fassung. Deutsche Adaptation der revidierten Fassung der Wechsler Memory Scale. Huber: Bern, 2000.
Rey A . L'examen clinique en psychologie. Presses Universitaires de France: Paris, 1964.
Helmstaedter C, Lendt M, Lux S . VLMT Verbaler Lern- und Merkfähigkeitstest. Beltz Test: Göttingen, 2001.
Aschenbrenner S, Tucha O, Lange KW . RWT Regensburger Wortflüssigkeits-Test. Hogrefe: Göttingen, 2000.
Horn W . L-P-S Leistungsprüfsystem, 2 Auflage. Göttingen: Hogrefe, 1983.
Aaronson NK, Ahmadzai S, Bergman B, Bullinger M, Cull A, Duez NJ et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376.
Spreen O, Strauss E . A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 2nd edn. Oxford University Press: New York, NY, 1998.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neimann PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 1974; 18: 295–304.
Booth-Jones M, Jacobsen PB, Ransom S, Soety E . Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant 2005; 36: 695–702.
van Dam FSAM, Schagen SB, Muller MJ, Boogert W, vd Wall E, Droogleever Fortuyn ME et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst 1998; 90: 210–218.
Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rijn D, Rodenhuis S et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol 2002; 13: 1387–1397.
Scherwath A, Mehnert A, Schleimer B, Schirmer L, Fehlauer F, Kreienberg R et al. Neuropsychological function on high-risk breast cancer survivors after stem-cell supported high-dose therapy versus standard-dose chemotherapy: evaluation of long-term treatment effects. Ann Oncol 2006; 17: 415–423.
Ahles T, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003; 12: 612–619.
Altundag K, Moussallem CD, Baptista MZ . Promotion of neurogenesis by human stem cells in high-risk breast cancer survivals after stem-cell supported high-dose therapy. Ann Oncol 2006; 17: 1465.
Acknowledgements
This research was funded by the German José Carreras Leukemia Foundation e.V.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulz-Kindermann, F., Mehnert, A., Scherwath, A. et al. Cognitive function in the acute course of allogeneic hematopoietic stem cell transplantation for hematological malignancies. Bone Marrow Transplant 39, 789–799 (2007). https://doi.org/10.1038/sj.bmt.1705663
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705663
Keywords
This article is cited by
-
Advancing Palliative Care Integration in Hematology: Building Upon Existing Evidence
Current Treatment Options in Oncology (2023)
-
Patient-reported cognitive function among hematopoietic stem cell transplant and cellular therapy patients: a scoping review
Quality of Life Research (2023)
-
What is known about palliative care in adult patients with allogeneic stem cell transplantation (allo-SCT)?
Annals of Hematology (2021)
-
Late cognitive outcomes among allogeneic stem cell transplant survivors: follow-up data from a 6-year longitudinal study
Supportive Care in Cancer (2021)
-
Predictors of the trajectory of cognitive functioning in the first 6 months after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2020)