Abstract
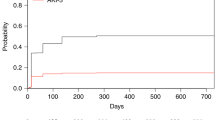
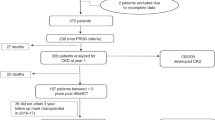
Acute renal failure (ARF) is an important complication after stem cell transplantation (SCT). We retrospectively analysed ARF in 363 recipients of allogeneic myeloablative SCT to identify incidence, risk factors, associated post-transplantation complications and mortality of ARF. ARF was graded as grade 0 (no ARF) to grade 3 (need for dialysis) according to creatinine, estimated glomerular filtration rate and need for dialysis. The incidence of severe renal failure (grades 2 and 3 combined) was 49.6% (180 of 363 patients). Hypertension present at SCT was identified as a risk factor for ARF (P=0.003). Despite this, survival of these patients was not different compared to patients without hypertension. Admission to the intensive care unit (ICU) was a post-transplantation complication significantly associated with ARF (P<0.001). Survival rate was highest in patients with ARF grade 0–1 and lowest in patients with grade 3 (P<0.001). However, after correction for complications associated with high mortality (admission to the ICU, thrombotic thrombocytopenic purpura, sinusoidal occlusion syndrome (SOS) and acute graft-versus-host disease) the significant difference in survival disappeared, showing that ARF without co-morbid conditions has a good prognosis, and ARF with co-morbid conditions has a poor prognosis. This poor prognosis is due to the presence of co-morbid conditions rather than development of ARF itself.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rubenfeld GD, Crawford SW . Withdrawing life support from mechanically ventilated recipients of bone marrow transplants: a case for evidence-based guidelines. Ann Intern Med 1996; 125: 625–633.
Mcdonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Venoocclusive disease of the liver and multiorgan failure after bone-marrow transplantation – a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB . Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion 2004; 44: 294–304.
Thomas ED, Storb R, Clift RA, Fefer A, Johnson L, Neiman PE et al. Bone-marrow transplantation (second of two parts). N Engl J Med 1975; 292: 895–902.
Meijer E, Boland GJ, Verdonck LF . Prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplants. Clin Microbiol Rev 2003; 16: 647–657.
Hahn T, Rondeau C, Shaukat A, Jupudy V, Miller A, Alam AR et al. Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant 2003; 32: 405–410.
Letourneau I, Dorval M, Belanger R, Legare M, Fortier L, Leblanc M . Acute renal failure in bone marrow transplant patients admitted to the intensive care unit. Nephron 2002; 90: 408–412.
Parikh CR, Coca SG . Acute renal failure in hematopoietic cell transplantation. Kidney Int 2006; 69: 430–435.
Zager RA, Madias NE, Harrington JT, Singh A, Perrone R, King A et al. Acute-renal-failure in the setting of bone-marrow transplantation. Kidney Int 1994; 46: 1443–1458.
Gruss E, Bernis C, Tomas JF, Garciacanton C, Figuera A, Motellon JL et al. Acute-renal-failure in patients following bone-marrow transplantation – prevalence, risk-factors and outcome. Am J Nephrol 1995; 15: 473–479.
Parikh CR, McSweeney P, Schrier RW . Acute renal failure independently predicts mortality after myeloablative allogeneic hematopoietic cell transplant. Kidney Int 2005; 67: 1999–2005.
Noel C, Hazzan M, Noel-Walter MP, Jouet JP . Renal failure and bone marrow transplantation. Nephrol Dial Transplant 1998; 13: 2464–2466.
Verdonck LF, Dekker AW, de Gast GC, van Kempen ML, Lokhorst HM, Nieuwenhuis HK . Allogeneic bone marrow transplantation with a fixed low number of T cells in the marrow graft. Blood 1994; 83: 3090–3096.
Manjunath G, Sarnak MJ, Levey AS . Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens 2001; 10: 785–792.
Hingorani SR, Guthrie K, Batchelder A, Schoch G, Aboulhosn N, Manchion J et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int 2005; 67: 272–277.
Miralbell R, Bieri S, Mermillod B, Helg C, Sancho G, Pastoors B et al. Renal toxicity after allogeneic bone marrow transplantation: The combined effects of total-body irradiation and graft-versus-host disease. J Clin Oncol 1996; 14: 579–585.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000; 96: 2062–2068.
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 1998; 92: 2303–2314.
Zager RA, Oquigley J, Zager BK, Alpers CE, Shulman HM, Gamelin LM et al. Acute renal-failure following bone-marrow transplantation – a retrospective study of 272 patients. Am J Kidney Dis 1989; 13: 210–216.
K/DOQI clinical practice guidelines on hypertension and anti hypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43: S14–S290.
Naeem N, Reed MD, Creger RJ, Youngner SJ, Lazarus HM . Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant 2006; 37: 119–133.
van der Plas RM, Schiphorst ME, Huizinga EG, Hene RJ, Verdonck LF, Sixma JJ et al. von Willebrand factor proteolysis is deficient in classic, but not in bone marrow transplantation-associated, thrombotic thrombocytopenic purpura. Blood 1999; 93: 3798–3802.
Ruutu T, Hermans J, Niederwieser D, Gratwohl A, Kiehl M, Volin L et al. Thrombotic thrombocytopenic purpura after allogeneic stem cell transplantation: a survey of the European group for blood and marrow transplantation (EBMT). Br J Haematol 2002; 118: 1112–1119.
Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 2002; 62: 566–573.
Acknowledgements
We thank Dr P Westers, Centre of Biostatistics University Utrecht, for expert statistical assistance. We are also grateful to MI Gerrits, J vd Giessen and Dr E Meijer (Department of Haematology, University Medical Centre Utrecht) for providing data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kersting, S., Koomans, H., Hené, R. et al. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant 39, 359–365 (2007). https://doi.org/10.1038/sj.bmt.1705599
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705599
Keywords
This article is cited by
-
Incidence of acute kidney injury after hematopoietic stem cell transplantation in children: a systematic review and meta-analysis
European Journal of Pediatrics (2023)
-
Acute kidney injury within 100 days post allogeneic hematopoietic cell transplantation is associated with increased risk of post-transplant complications and poor transplant outcomes
Bone Marrow Transplantation (2022)
-
Predicting the risk of acute kidney injury after hematopoietic stem cell transplantation: development of a new predictive nomogram
Scientific Reports (2022)
-
Acute kidney injury in cancer patients
Clinical and Experimental Nephrology (2022)
-
Pediatric onco-nephrology: time to spread the word
Pediatric Nephrology (2021)