Abstract
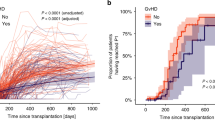
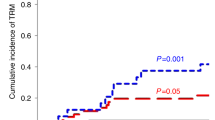
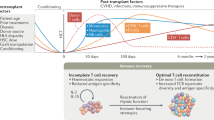
Delayed and/or insufficient T cell recovery post hematopoietic stem cell transplantation (HSCT) leads to an increased risk of morbidity and mortality. We evaluated thymic function and its association with T cell regeneration post HSCT and identified factors involved in the process among pediatric stem cell transplant recipients. T cell regeneration in 66 pediatric patients was prospectively followed by naive T cell phenotyping, measuring of T cell receptor excision circles (TRECs) and expression of Foxp3 by regulatory T cells for the first 18 months post HSCT. TRECs were lower pre-HSCT in children with a malignant than non-malignant primary disease or immunosuppressed controls (P=0.001). Naive T lymphocyte reconstitution and thymic recovery were slow in the recipients of allogeneic stem cell grafts post HSCT. Infections caused by herpesviruses had a prognostic impact on mortality. Children with low TRECs had a high mortality (P=0.05) and low TRECs were also associated with extensive chronic graft-versus-host disease from 6 months onwards. Low amount of Foxp3 pre-HSCT was associated with an increased mortality post HSCT (P=0.03). Our study indicates an association between impaired T cell regeneration and thymic dysfunction and the clinical post transplant complications in pediatric allogeneic stem cell transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weinberg K, Annett G, Kashyap A, Lenarsky C, Forman SJ, Parkman R . The effect of thymic function on immunocompetence following bone marrow transplantation. Biol Blood Marrow Transplant 1995; 1: 18–23.
Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G et al. Immune reconstitution following T cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant 1997; 3: 65–75.
Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R . Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4T cell counts. Am J Hematol 1997; 54: 131–138.
Balduzzi A, Gooley T, Anasetti C, Sanders JE, Martin PJ, Petersdorf EW et al. Unrelated donor marrow transplantation in children. Blood 1995; 86: 3247–3256.
Ochs L, Shu XO, Miller J, Enright H, Wagner J, Filipovich A et al. Late infections after allogeneic bone marrow transplantations: comparison of incidence in related and unrelated donor transplant recipients. Blood 1995; 86: 3979–3986.
Mackall CL, Granger L, Sheard MA, Cepeda R, Gress RE . T cell regeneration after bone marrow transplantation: differential CD45 isoform expression on thymic-derived versus thymic-independent progeny. Blood 1993; 82: 2585–2594.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332: 143–149.
Mackall CL, Hakim FT, Gress RE . T cell regeneration: all repertoires are not created equal. Immunol Today 1997; 18: 245–251.
Mackall CL, Gress RE . Pathways of T cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev 1997; 157: 61–72.
Heitger A, Neu N, Kern H, Panzer-Grumayer ER, Greinix H, Nachbaur D et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood 1997; 90: 850–857.
Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE . Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol 1996; 156: 4609–4616.
Chung B, Barbara-Burnham L, Barsky L, Weinberg K . Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood 2001; 98: 1601–1606.
Clark FJ, Gregg R, Piper K, Dunnion D, Freeman L, Griffiths M et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25 high regulatory T cells. Blood 2004; 103: 2410–2416.
Hazenberg MD, Otto SA, de Pauw ES, Roelofs H, Fibbe WE, Hamann D et al. T cell receptor excision circle and T cell dynamics after allogeneic stem cell transplantation are related to clinical events. Blood 2002; 99: 3449–3453.
Lewin SR, Heller G, Zhang L, Rodrigues E, Skulsky E, van den Brink MR et al. Direct evidence for new T cell generation by patients after either T cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood 2002; 100: 2235–2242.
Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood 2001; 97: 1458–1466.
Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396: 690–695.
Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG . Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation 2002; 73: 1154–1158.
Clave E, Rocha V, Talvensaari K, Busson M, Douay C, Appert ML et al. Prognostic value of pretransplantation host thymic function in HLA-identical sibling hematopoietic stem cell transplantation. Blood 2005; 105: 2608–2613.
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood 2005; 106: 2903–2911.
Suri-Payer E, Amar AZ, Thornton AM, Shevach EM . CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol 1998; 160: 1212–1218.
Hori S, Nomura T, Sakaguchi S . Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299: 1057–1061.
Chai JG, Xue SA, Coe D, Addey C, Bartok I, Scott D et al. Regulatory T cells, derived from naive CD4+CD25− T cells by in vitro Foxp3 gene transfer, can induce transplantation tolerance. Transplantation 2005; 79: 1310–1316.
Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood 2004; 104: 2187–2193.
Talvensaari K, Clave E, Douay C, Rabian C, Garderet L, Busson M et al. A broad T cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood 2002; 99: 1458–1464.
Chen X, Barfield R, Benaim E, Leung W, Knowles J, Lawrence D et al. Prediction of T cell reconstitution by assessment of T cell receptor excision circle before allogeneic hematopoietic stem cell transplantation in pediatric patients. Blood 2005; 105: 886–893.
Svaldi M, Lanthaler AJ, Dugas M, Lohse P, Pescosta N, Straka C et al. T cell receptor excision circles: a novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br J Haematol 2003; 122: 795–801.
Petridou E, Klimentopoulou AE, Moustaki M, Kostrikis LG, Hatzakis A, Trichopoulos D . Recent thymic emigrants and prognosis in T- and B-cell childhood hematopoietic malignancies. Int J Cancer 2002; 101: 74–77.
Eyrich M, Wollny G, Tzaribaschev N, Dietz K, Brugger D, Bader P et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant 2005; 11: 194–205.
Foot AB, Potter MN, Donaldson C, Cornish JM, Wallington TB, Oakhill A et al. Immune reconstitution after BMT in children. Bone Marrow Transplant 1993; 11: 7–13.
de Vries E, van Tol MJ, van den Bergh RL, Waaijer JL, ten Dam MM, Hermans J et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant 2000; 25: 267–275.
Paulin T, Ringden O, Nilsson B . Immunological recovery after bone marrow transplantation: role of age, graft-versus-host disease, prednisolone treatment and infections. Bone Marrow Transplant 1987; 1: 317–328.
Fujimaki K, Maruta A, Yoshida M, Kodama F, Matsuzaki M, Fujisawa S et al. Immune reconstitution assessed during five years after allogeneic bone marrow transplantation. Bone Marrow Transplant 2001; 27: 1275–1281.
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant 1995; 16: 413–425.
Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001; 97: 3380–3389.
Acknowledgements
We thank Inna Sareneva and Noora Alakulppi MSc for technical assistance. This study was supported by the Nona and Kullervo Väre Foundation and the Finnish Cultural Foundation and the Sigrid Juselius Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olkinuora, H., Talvensaari, K., Kaartinen, T. et al. T cell regeneration in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant 39, 149–156 (2007). https://doi.org/10.1038/sj.bmt.1705557
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705557
Keywords
This article is cited by
-
Beyond monogenetic rare variants: tackling the low rate of genetic diagnoses in predominantly antibody deficiency
Cellular & Molecular Immunology (2021)
-
Stem cell source-dependent reconstitution of FOXP3+ T cells after pediatric SCT and the association with allo-reactive disease
Bone Marrow Transplantation (2013)
-
Multivariate analyses of immune reconstitution in children after allo-SCT: risk-estimation based on age-matched leukocyte sub-populations
Bone Marrow Transplantation (2010)
-
Plasma levels of IL-7 and IL-15 in the first month after myeloablative BMT are predictive biomarkers of both acute GVHD and relapse
Bone Marrow Transplantation (2010)
-
Association of IL-10 and IL-10Rβ gene polymorphisms with graft-versus-host disease after haematopoietic stem cell transplantation from an HLA-identical sibling donor
BMC Immunology (2009)