Abstract
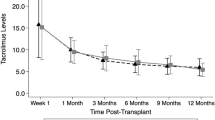
Tacrolimus (Prograf®, FK506, Fujisawa Healthcare) is a widely used immunosuppressive agent that is used both for the prevention and treatment of solid organ transplant rejection as well as for the prevention and treatment of graft-versus-host disease after allogeneic blood and marrow transplant. Oral preparations of tacrolimus are commercially available in 0.5, 1 and 5 mg gelatin capsules. Previously, only a 0.5 mg/ml oral suspension has been demonstrated to be stable for use in pediatric patients. On our bone marrow transplant service, we found that using this concentration of tacrolimus led to confusion, with patients and their caregivers confusing milligrams and milliliters, thus increasing errors with this formulation. We postulated that a 1 mg/ml oral formulation of tacrolimus would decrease the potential for medication errors. Our findings support new stability information of approximately 4 months for an extemporaneous oral suspension of tacrolimus at a concentration of 1 mg/ml.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Goto T, Kino T, Hatanaka H, Okuhara M, Kohsaka M, Aoki H et al. FK506: historical perspectives. Transplant Proc 1991; 23: 2713–2717.
Prograf package insert. Fujisawa: Deerfield, IL, April 1994.
Jacobson PA, Johnson CE, West NJ, Foster JA . Stability of tacrolimus in an extemporaneously compounded oral liquid. Am J Health Syst Pharm 1997; 54: 178–180.
Taormina D, Abdallah HY, Venkataramanan R, Logue L, Burckart GJ, Ptachcinski RJ et al. Stability and sorption of FK506 in 5% dextrose injection and 0.9% sodium chloride injection in glass, polyvinyl chloride and polyolefin containers. Am J Hosp Pharm 1992; 49: 119–122.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elefante, A., Muindi, J., West, K. et al. Long-term stability of a patient-convenient 1 mg/ml suspension of tacrolimus for accurate maintenance of stable therapeutic levels. Bone Marrow Transplant 37, 781–784 (2006). https://doi.org/10.1038/sj.bmt.1705320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705320