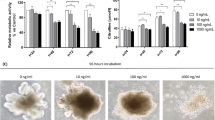
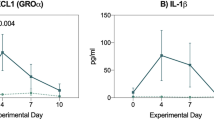
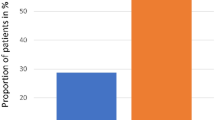
Summary:
Severe mucositis is a common cause of morbidity in hematopoietic stem cell transplant (HSCT) recipients. Glutamine has been shown to reduce mucositis in children receiving chemotherapy. Patients were randomized in a double-blind manner to receive glutamine or glycine at a dose of 2 g/m2/dose (maximum dose 4 g) twice daily until 28 days post transplant or discharge if sooner. Mucositis was graded by use of a modified Walsh scale. A total of 120 children were evaluable: 57 children received glutamine and 63 received glycine. The mean mucositis score was 3.0±0.3 vs 3.9±0.4 (P=0.07) in the glutamine and glycine groups, respectively. The glutamine group demonstrated a reduction in mean number of days of intravenous narcotics use (12.1±1.5 vs 19.3±2.8 in the glycine group, P=0.03) and total parenteral nutrition (17.3±1.7 vs 27.3±3.6 in glycine group, P=0.01). There was no statistically significant difference in toxicity between the two groups. Glutamine appears to be safe and beneficial in reducing the severity of mucositis. Strong consideration should be given to include oral glutamine supplementation as a routine part of supportive care of SCT patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Demarosi F, Bez C, Carrassi A . Prevention and treatment of chemo- and radiotherapy-induced oral mucositis. Minerva Stomatol 2002; 51: 173–186.
Stiff P . Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant 2000; 27: S3–S11.
Bellm LA, Epstein JB, Rose-Ped AM et al. Assessment of various topical oral formulations by bone marrow transplant recipients. Oral Oncol 2001; 37: 42–49.
Sonis ST, Elting LS, Keefe D et al. Perspectives on cancer therapy-induced mucosal injury. Cancer 2004; 100 (Suppl. 9): 1995–2025.
Köstler WJ, Hejna M, Wenzel C et al. Oral mucositis complicating chemotherapy and/or radiotherapy: options for prevention and treatment. CA Cancer J Clin 2001; 51: 290–315.
Gamis A, Call S, Cook L et al. A multi-institutional, randomized, double blind, placebo-controlled study of selective oropharyngeal decontamination (SD) to reduce mucositis (MCS) in pediatric BMT patients. Blood 1996; 88: 1640a.
Loprinizi CL, Ghosh C, Camorian J et al. Phase III controlled evaluation of sucralfate to alleviate stomatitis in patients receiving fluorouracil-based chemotherapy. J Clin Oncol 1997; 15: 1235–1238.
Makkonen TA, Bostrom P, Vilja P et al. Sucralfate mouth washing in the prevention of radiation-induced mucositis: a placebo-controlled double-blind randomized study. Int J Radiat Oncol Biol Phys 1994; 37: 275–279.
Shenep JL, Kalwinshky DK, Hutson PR et al. Efficacy of oral sucralfate suspension in prevention and treatment of chemotherapy-induced mucositis. J Pediatr 1988; 113: 758–763.
Karthaus M, Rosenthal C, Huebner G et al. Effect of topical oral G-CSF on oral mucositis: a randomised placebo-controlled trial. Bone Marrow Transplant 1998; 22: 781–785.
Bez C, Demarosi F, Sardella A et al. GM-CSF mouth rinses in the treatment of severe oral mucositis: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 1999; 88: 311–315.
Ovilla-Martinez R, Rubio ME, Borbolla JR . GM-CSF mouthwashes as treatment for mucositis in BMT patients. Blood 1994; 82: Abstract 2853.
Carnel SB, Blakeslee DB, Oswald SG, Barnes M . Treatment of radiation-and chemotherapy-induced stomatitis. Otolaryngol Head Neck Surg 1990; 102: 326–330.
Leque FG, Parzuchowski JB, Farinacci GC et al. Clinical evaluation of MGI 209, an anesthetic, film-forming agent for relief from painful oral ulcers associated with chemotherapy. J Clin Oncol 1990; 10: 1963–1968.
Fahlke J, Ridwelski K, Lippert H . High-dose therapy with combined 5-fluorouracil and folinic acid with and without amifostine in the treatment of patients with metastatic colorectal carcinoma. Int J Colorectal Dis 1999; 14: 128–130.
Gabriel DA, Shea T, Wiley J et al. Use of amifostine to reduce mucositis following total body irradiation based autotransplants for lymphoma. Proc Am Soc Clin Oncol 2000; 19: 69a.
Spielberger R, Stiff P, Bensinger W et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 2004; 351: 2590–2598.
Van der Hulst RR, Van Kreel BK, Von Meyenfeldt MF et al. Glutamine and the preservation of gut integrity. Lancet 1993; 341: 1363–1365.
Anderson PM, Schroeder G, Subitz KM . Oral glutamine reduces duration and severity of stomatitis after chemotherapy. J Pediatr Hematol Oncol 1997; 19: 379.
DTCD, NCI, NIH, DHHS. Cancer Therapy Evaluation Program: Common Toxicity Criteria, Version 3.0. (http://ctep.info.nih.gov/CTC3/ctc.htm). 1998.
Walsh L, Hill G, Seymour G, Roberts A . A scoring system for the quantitative evaluation of oral mucositis during bone marrow transplantation. Spec Care Dentist 1999; 10: 190–195.
Bergstrom J, Furst P, Moore LO et al. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 1974; 36: 393–397.
Lacey JM, Wilmore DW . Is glutamine a conditionally essential amino acid? Nutr Rev 1990; 48: 297–303.
Souba WW, Klimberg VS, Plumley DA et al. The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res 1990; 48: 383–391.
Askanazi J, Carpentier YA, Michelsen CB et al. Muscle and plasma amino acids following injury. Ann Surg 1980; 192: 78–85.
Vinnars E, Bergstrom J, Furst P . Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Ann Surg 1993; 182: 665.
Parry-Billings M, Evans J, Calder PC et al. Does glutamine contribute to immunosuppression after major burns? Lancet 1990; 336: 523–525.
Roth E, Funovics J, Mulhbacher F et al. Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1982; 1: 25–41.
Klimberg VS, Salloum RM, Kasper M et al. Oral glutamine accelerates healing of the small intestine and improves outcome after whole abdominal radiation. Arch Surg 1990; 125: 1040–1045.
Burke DJ, Alverdy JC, Aoys E et al. Glutamine supplemented TPN improves gut immune function. Arch Surg 1989; 124: 1396–1399.
Hammarqvist F, Wernerman J, Ali R et al. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis and improves nitrogen balance. Ann Surg 1989; 211: 455–461.
Hardy G, Bevan SJ . Stability of glutamine in parenteral feeding solutions. Lancet 1993; 342: 186.
Lowe DK, Benfell K, Smith RJ et al. Safety of glutamine-enriched parenteral nutrient solutions in humans. Am J Clin Nutr 1990; 52: 1101–1106.
Piccirillo N, De Matteis S, Laurenti L et al. Glutamine-enriched parenteral nutrition after autologous peripheral blood stem cell transplantation: effects on immune reconstitution and mucositis. Haematologica 2003; 88: 192–200.
Sax HC . Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double-blind, controlled study. JPEN J Parenter Enteral Nutr 1992; 16: 589–590.
Schloerb PR, Amare M . Total parenteral nutrition with glutamine in bone marrow transplantation and other clinical applications (a randomized double-blind study). JPEN J Parenter Enteral Nutr 1993; 17: 407–413.
Schloerb PR, Skikne BS . Oral and parenteral glutamine in bone marrow transplantation: a randomized, double-blind study. JPEN J Parenter Enteral Nutr 1999; 23: 117–122.
Souba WW . Total parenteral nutrition with glutamine in bone marrow transplantation and other clinical applications. JPEN J Parenter Enteral Nutr 1993; 17: 403.
Stehle P, Zander J, Mertes N et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1989; 1: 231–232.
van Zaanen HC, van der Lelie H, Timmer JG et al. Parenteral glutamine dipeptide supplementation does not ameliorate chemotherapy-induced toxicity. Cancer 1994; 70: 732–735.
Ziegler TR, Bye RL, Persinger RL et al. Glutamine-enriched parenteral nutrition increases circulating lymphocytes after bone marrow transplantation. JPEN J Parenter Enteral Nutr 1994; 18: 17S.
Ziegler TR, Young LS, Benfell K et al. Clinical and metabolic efficacy of glutamine-supplemented parenteral nutrition after bone marrow transplantation. A randomized, double blind, controlled study. Ann Intern Med 1992; 116: 821–828.
MacBurney M, Young LS, Ziegler TR et al. A cost evaluation of glutamine supplemented parenteral nutrition in adult bone marrow transplant patients. J Am Diet Assoc 1994; 94: 1263–1266.
Anderson PM, Ramsay NK, Shu XO et al. Effect of low-dose oral glutamine on painful stomatitis during bone marrow transplantation. Bone Marrow Transplant 1998; 22: 339–344.
Cockerham MB, Weinberger BB, Lerchie SB . Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother 2000; 34: 300–303.
Coghlin Dickson TM, Wong RM, Offrin RS et al. Effect of oral glutamine supplementation during bone marrow transplantation. JPEN J Parenter Enteral Nutr 2000; 24: 61–66.
Daniele B, Perrone F, Gallo C et al. Oral glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut 2001; 48: 28–33.
Jebb SA, Osborne RJ, Maughan TS et al. 5-Fluorouracil and folinic acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer 1994; 70: 732–735.
Skubitz KM, Anderson PM . Oral glutamine to prevent chemotherapy induced stomatitis: a pilot study. J Lab Clin Med 1996; 127: 223–228.
Ziegler TR, Bye RL, Persinger RL et al. Effects of glutamine supplementation on circulating lymphocytes after bone marrow transplantation: a pilot study. Am J Med Sci 1998; 315: 4–10.
Acknowledgements
Participating Centers of the Pediatric Blood and Marrow Transplant Consortium are as follows: UT Southwestern Medical Center at Dallas; Nemours Children's Clinic – Jacksonville; Children's Hospital Medical Center of Akron; Children's National Medical Center; Columbia University Medical Center/Children's Hospital of New York; Long Island Jewish Health System; University of South Carolina; Roswell Park Cancer Institute; Texas Transplant Institute; University of Calgary; Rainbow Babies & Children's Hospital; Washington University Medical Center. This work was supported by The Children's Cancer Fund, Wipe Out Kids Cancer, and the Nemours Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Aquino, V., Harvey, A., Garvin, J. et al. A double-blind randomized placebo-controlled study of oral glutamine in the prevention of mucositis in children undergoing hematopoietic stem cell transplantation: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant 36, 611–616 (2005). https://doi.org/10.1038/sj.bmt.1705084
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705084
Keywords
This article is cited by
-
MASCC/ISOO clinical practice guidelines for the management of mucositis: sub-analysis of current interventions for the management of oral mucositis in pediatric cancer patients
Supportive Care in Cancer (2021)
-
Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines—part 1: vitamins, minerals, and nutritional supplements
Supportive Care in Cancer (2019)
-
A systematic review of integrative clinical trials for supportive care in pediatric oncology: a report from the International Society of Pediatric Oncology, T&CM collaborative
Supportive Care in Cancer (2018)
-
Elemental diet plus glutamine for the prevention of mucositis in esophageal cancer patients receiving chemotherapy: a feasibility study
Supportive Care in Cancer (2016)
-
Systematic review of natural agents for the management of oral mucositis in cancer patients
Supportive Care in Cancer (2013)