Summary:
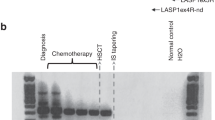
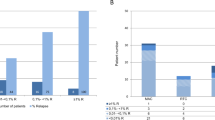
We performed real-time quantitative polymerase chain reaction (RQ-PCR) in peripheral blood (PB) and/or bone marrow (BM) samples collected pre- and post transplant from 23 recipient–donor pairs receiving allogeneic stem cell transplantation (allo-SCT) for follicular lymphoma (FL). Of 23 donors, 11 had a PB and/or BM sample positive for t(14;18) (BCL2/IGH fusion) at low levels (<one t(14;18) cell in 10K total cells). Recipients from donors with (n=11) and those without (n=12) detectable t(14:18) cells were similar in age, sex, and disease status pretransplant. No differences in the incidence of graft-versus-host-disease (GVHD), delayed engraftment, relapse rate, disease-free survival and overall survival were identified between the groups. Two recipients without detectable t(14;18) cells pre-transplant showed detectable t(14;18) cells at 2 and 11 years after receiving grafts from donors with t(14:18) cells. Neither patient developed FL 1.5 and 2 years after the emergence of t(14;18) cells. Although the sample size is relatively small, our findings suggest that individuals carrying t(14;18) cells may not be excluded as donors given the lack of an association of t(14;18) detected in donors with adverse clinical outcome. It may be necessary to screen for the donor's t(14;18) status before using t(14;18) for monitoring minimal residual disease by RQ-PCR to exclude the possibility of confounding donor's t(14;18) clone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Turner GE, Ross FM, Krajewski AS . Detection of t(14;18) in British follicular lymphoma using cytogenetics, Southern blotting and the polymerase chain reaction. Br J Haematol 1995; 89: 223–225.
Pappa VI, Wilkes S, Salam A et al. Use of the polymerase chain reaction and direct sequencing analysis to detect cells with the t(14;18) in autologous bone marrow from patients with follicular lymphoma, before and after in vitro treatment. Bone Marrow Transplant 1998; 22: 553–558.
Aster JC, Longtine JA . Detection of BCL2 rearrangements in follicular lymphoma. Am J Pathol 2002; 160: 759–763.
Yasukawa M, Bando S, Dolken G et al. Low frequency of BCL-2/J(H) translocation in peripheral blood lymphocytes of healthy Japanese individuals. Blood 2001; 98: 486–488.
Ladetto M, Drandi D, Compagno M et al. PCR-detectable nonneoplastic Bcl-2/IgH rearrangements are common in normal subjects and cancer patients at diagnosis but rare in subjects treated with chemotherapy. J Clin Oncol 2003; 21: 1398–1403.
Ji W, Qu GZ, Ye P et al. Frequent detection of bcl-2/JH translocations in human blood and organ samples by a quantitative polymerase chain reaction assay. Cancer Res 1995; 55: 2876–2882.
Summers KE, Goff LK, Wilson AG et al. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol 2001; 19: 420–424.
Tsimberidou AM, Jiang Y, Ford RJ et al. Quantitative real-time polymerase chain reaction for detection of circulating cells with t(14;18) in volunteer blood donors and patients with follicular lymphoma. Leuk Lymphoma 2002; 43: 1589–1598.
Fuscoe JC, Setzer RW, Collard DD, Moore MM . Quantification of t(14;18) in the lymphocytes of healthy adult humans as a possible biomarker for environmental exposures to carcinogens. Carcinogenesis 1996; 17: 1013–1020.
Liu Y, Hernandez AM, Shibata D, Cortopassi GA . BCL2 translocation frequency rises with age in humans. Proc Natl Acad Sci USA 1994; 91: 8910–8914.
Dolken G, Illerhaus G, Hirt C, Mertelsmann R . BCL-2/JH rearrangements in circulating B cells of healthy blood donors and patients with nonmalignant diseases. J Clin Oncol 1996; 14: 1333–1344.
Finke J . The role of stem cell transplantation in the treatment of follicular lymphoma. Semin Cancer Biol 2003; 13: 233–239.
van Besien K, Loberiza Jr FR, Bajorunaite R et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood 2003; 102: 3521–3529.
Juckett M, Rowlings P, Hessner M et al. T cell-depleted allogeneic bone marrow transplantation for high-risk non-Hodgkin's lymphoma: clinical and molecular follow-up. Bone Marrow Transplant 1998; 21: 893–899.
Stein RS, Greer JP, Goodman S et al. High-dose therapy with autologous or allogeneic transplantation as salvage therapy for small cleaved cell lymphoma of follicular center cell origin. Bone Marrow Transplant 1999; 23: 227–233.
Chang CC, Bredeson C, Juckett M et al. Tumor load in patients with follicular lymphoma post stem cell transplantation may correlate with clinical course. Bone Marrow Transplant 2003; 32: 287–291.
Rosenblum MD, Drobyski WR, Keever-Taylor C, Chang CC . Concurrent presence of both patient and donor t(14;18) in a follicular lymphoma patient after undergoing allogeneic BMT: implications for minimal residual disease detection post-transplant. Bone Marrow Transplant 2003; 31: 947–949.
Dolken L, Schuler F, Dolken G . Quantitative detection of t(14;18)-positive cells by real-time quantitative PCR using fluorogenic probes. Biotechniques 1998; 25: 1058–1064.
Hirt C, Dolken G . Quantitative detection of t(14;18)-positive cells in patients with follicular lymphoma before and after autologous bone marrow transplantation. Bone Marrow Transplant 2000; 25: 419–426.
Ladetto M, Sametti S, Donovan JW et al. A validated real-time quantitative PCR approach shows a correlation between tumor burden and successful ex vivo purging in follicular lymphoma patients. Exp Hematol 2001; 29: 183–193.
Mandigers CM, Meijerink JP, Mensink EJ et al. Lack of correlation between numbers of circulating t(14;18)-positive cells and response to first-line treatment in follicular lymphoma. Blood 2001; 98: 940–944.
Keever-Taylor CA, Craig A, Molter M et al. Complement-mediated T-cell depletion of bone marrow: comparison of T10B9.1A-31 and Muromonab-Orthoclone OKT3. Cytotherapy 2001; 3: 467–481.
Viardot A, Barth TF, Moller P et al. Cytogenetic evolution of follicular lymphoma. Semin Cancer Biol 2003; 13: 183–190.
Caporaso N, Marti GE, Goldin L . Perspectives on familial chronic lymphocytic leukemia: genes and the environment. Semin Hematol 2004; 41: 201–206.
Mandigers CM, Meijerink JP, Raemaekers JM et al. Graft-versus-lymphoma effect of donor leucocyte infusion shown by real-time quantitative PCR analysis of t(14;18). Lancet 1998; 352: 1522–1523.
Sarris AH, Jiang Y, Tsimberidou AM et al. Quantitative real-time polymerase chain reaction for monitoring minimal residual disease in patients with advanced indolent lymphomas treated with rituximab, fludarabine, mitoxantrone, and dexamethasone. Semin Oncol 2002; 29 (1 Suppl. 2): 48–55.
Summers KE, Davies AJ, Matthews J et al. The relative role of peripheral blood and bone marrow for monitoring molecular evidence of disease in follicular lymphoma by quantitative real-time polymerase chain reaction. Br J Haematol 2002; 118: 563–566.
Jenner MJ, Summers KE, Norton AJ et al. JH probe real-time quantitative polymerase chain reaction assay for Bcl-2/IgH rearrangements. Br J Haematol 2002; 118: 550–558.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGregor, D., Keever-Taylor, C., Bredeson, C. et al. The implication of follicular lymphoma patients receiving allogeneic stem cell transplantation from donors carrying t(14;18)-positive cells. Bone Marrow Transplant 35, 1049–1054 (2005). https://doi.org/10.1038/sj.bmt.1704969
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704969