Summary:
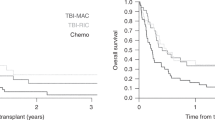
Efficacy of reduced-intensity stem-cell transplantation (RIST) for acute lymphoblastic leukemia (ALL) was investigated in 33 patients (median age, 55 years). RIST sources comprised 20 HLA-identical related donors, five HLA-mismatched related, and eight unrelated donors. Six patients had undergone previous transplantation. Disease status at RIST was first remission (n=13), second remission (n=6), and induction failure or relapse (n=14). All patients tolerated preparatory regimens and achieved neutrophil engraftment (median, day 12.5). Acute and chronic graft-versus-host disease (GVHD) developed in 45 and 64%, respectively. Six patients received donor lymphocyte infusion (DLI), for prophylaxis (n=1) or treatment of recurrent ALL (n=5). Nine patients died of transplant-related mortality, with six deaths due to GVHD. The median follow-up of surviving patients was 11.6 months (range, 3.5–37.3 months). The 1-year relapse-free and overall survival rates were 29.8 and 39.6%, respectively. Of the 14 patients transplanted in relapse, five remained relapse free for longer than 6 months. Cumulative rates of progression and progression-free mortality at 3 years were 50.9 and 30.4%, respectively. These findings suggest the presence of a graft-versus-leukemia effect for ALL. RIST for ALL is worth considering for further evaluation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Giona F, Testi AM, Annino L et al. Treatment of primary refractory and relapsed acute lymphoblastic leukaemia in children and adults: the GIMEMA/AIEOP experience. Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto. Associazione Italiana Ematologia ed Ocologia Pediatrica. Br J Haematol 1994; 86: 55–61.
Herzig RH, Bortin MM, Barrett AJ et al. Bone-marrow transplantation in high-risk acute lymphoblastic leukaemia in first and second remission. Lancet 1987; 1: 786–789.
Butturini A, Gale RP . Chemotherapy versus transplantation in acute leukaemia. Br J Haematol 1989; 72: 1–8.
Horowitz MM, Messerer D, Hoelzer D et al. Chemotherapy compared with bone marrow transplantation for adults with acute lymphoblastic leukemia in first remission. Ann Intern Med 1991; 115: 13–18.
Fiere D, Lepage E, Sebban C et al. Adult acute lymphoblastic leukemia: a multicentric randomized trial testing bone marrow transplantation as postremission therapy. The French Group on Therapy for Adult Acute Lymphoblastic Leukemia. J Clin Oncol 1993; 11: 1990–2001.
Avivi I, Goldstone AH . Bone marrow transplant in Ph+ ALL patients. Bone Marrow Transplant 2003; 31: 623–632.
Slavin S, Naparstek E, Nagler A et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood 1996; 87: 2195–2204.
Ferster A, Bujan W, Mouraux T et al. Complete remission following donor leukocyte infusion in ALL relapsing after haploidentical bone marrow transplantation. Bone Marrow Transplant 1994; 14: 331–332.
Horowitz MM, Gale RP, Sondel PM et al Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Appelbaum FR . Graft versus leukemia (GVL) in the therapy of acute lymphoblastic leukemia (ALL). Leukemia 1997; 11 (Suppl. 4): S15–S17.
Cornelissen JJ, Carston M, Kollman C et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood 2001; 97: 1572–1577.
Kolb HJ, Schattenberg A, Goldman JM et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia. Blood 1995; 86: 2041–2050.
Collins Jr RH, Goldstein S, Giralt S et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant 2000; 26: 511–516.
Slavin S, Nagler A, Naparstek E et al Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998; 91: 756–763.
Giralt S, Thall PF, Khouri I et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 2001; 97: 631–637.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Bornhauser M, Thiede C, Platzbecker U et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res 2001; 7: 2254–2262.
Niederwieser D, Maris M, Shizuru JA et al Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood 2003; 101: 1620–1629.
Maris MB, Niederwieser D, Sandmaier BM et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood 2003; 102: 2021–2030.
Rezvani K, Lalancette M, Szydlo R et al. Non-myeloablative stem cell transplant (NMSCT) in AML, ALL and MDS: disappointing outcome for patients with advanced phase disease. Blood 2000; 96: 479a.
Arnold R, Massenkeil G, Bornhauser M et al. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia 2002; 16: 2423–2428.
Martino R, Giralt S, Caballero MD et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica 2003; 88: 555–560.
Michallet M, Bilger K, Garban F et al. Allogeneic hematopoietic stem-cell transplantation after nonmyeloablative preparative regimens: impact of pretransplantation and posttransplantation factors on outcome. J Clin Oncol 2001; 19: 3340–3349.
Ruiz-Arguelles GJ, Gomez-Almaguer D, Ruiz-Arguelles A et al. Results of an outpatient-based stem cell allotransplant program using nonmyeloablative conditioning regimens. Am J Hematol 2001; 66: 241–244.
Childs R, Chernoff A, Contentin N et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 2000; 343: 750–758.
Bacigalupo A . Second EBMT Workshop on reduced intensity allogeneic hemopoietic stem cell transplants (RI-HSCT). Bone Marrow Transplant 2002; 29: 191–195.
Bacigalupo A . Third EBMT/AMGEN Workshop on reduced-intensity conditioning allogeneic haemopoietic stem cell transplants (RIC-HSCT), and panel consensus. Bone Marrow Transplant 2004; 33: 691–696.
Glucksberg H, Storb R, Fefer A et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304.
Przepiorka D, Weisdorf D, Martin P et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Weisdorf DJ, Nesbit ME, Ramsay NK et al. Allogeneic bone marrow transplantation for acute lymphoblastic leukemia in remission: prolonged survival associated with acute graft-versus-host disease. J Clin Oncol 1987; 5: 1348–1355.
Kersey JH, Weisdorf D, Nesbit ME et al. Comparison of autologous and allogeneic bone marrow transplantation for treatment of high-risk refractory acute lymphoblastic leukemia. N Engl J Med 1987; 317: 461–467.
Bader P, Hancock J, Kreyenberg H et al. Minimal residual disease (MRD) status prior to allogeneic stem cell transplantation is a powerful predictor for post-transplant outcome in children with ALL. Leukemia 2002; 16: 1668–1672.
Doney K, Fisher LD, Appelbaum FR et al. Treatment of adult acute lymphoblastic leukemia with allogeneic bone marrow transplantation. Multivariate analysis of factors affecting acute graft-versus-host disease, relapse, and relapse-free survival. Bone Marrow Transplant 1991; 7: 453–459.
Forman SJ, Schmidt GM, Nademanee AP et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. J Clin Oncol 1991; 9: 1570–1574.
Bader P, Klingebiel T, Schaudt A et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 1999; 13: 2079–2086.
Lonnqvist B, Brune M, Ljungman P . Lymphoblastoid human interferon and low dose IL-2 combined with donor lymphocyte infusion as therapy of a third relapse of CML – a case report. Bone Marrow Transplant 1996; 18: 241–242.
Fukuda T, Hackman RC, Guthrie KA et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003; 102: 2777–2785.
Mielcarek M, Martin PJ, Leisenring W et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 2003; 102: 756–762.
Fukuda T, Boeckh M, Carter RA et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 2003; 102: 827–833.
Acknowledgements
We are grateful to Drs Takanori Teshima, Naoki Kobayashi, Takashi Ashida, Atsushi Woke, Issei Hatanaka and Shinji Nakao for their cooperation and detailed descriptions of their cases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamaki, T., Kami, M., Kanda, Y. et al. Reduced-intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant 35, 549–556 (2005). https://doi.org/10.1038/sj.bmt.1704776
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704776
Keywords
This article is cited by
-
Total body irradiation versus busulfan based intermediate intensity conditioning for stem cell transplantation in ALL patients >45 years—a registry-based study by the Acute Leukemia Working Party of the EBMT
Bone Marrow Transplantation (2023)
-
Comparison of reduced-intensity conditioning regimens in patients with acute lymphoblastic leukemia >45 years undergoing allogeneic stem cell transplantation—a retrospective study by the Acute Leukemia Working Party of EBMT
Bone Marrow Transplantation (2020)
-
Treatment of Older Patients with Acute Lymphoblastic Leukaemia
Drugs & Aging (2018)
-
Reduced Intensity Conditioning Allogeneic Hematopoietic Stem Cell Transplantation for Acute Lymphoblastic Leukemia; Current Evidence, and Improving Outcomes Going Forward
Current Hematologic Malignancy Reports (2018)
-
Who Should Receive a Transplant for Acute Lymphoblastic Leukaemia?
Current Hematologic Malignancy Reports (2017)