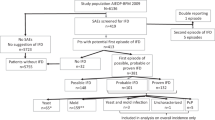
Summary:
We conducted a nationwide survey to define incidence of deep fungal infections and fungal prophylaxis practices after HSCT. In all, 63 institutions responded. Total number of in-patient transplantations was 935: 367 autologous, 414 allogeneic myeloablative, and 154 allogeneic reduced-intensity (RIST) (n=154). Number of patients who were cared for in a clean room at transplant was 261 (71%) in autologous, 409 (99%) in conventional and 93 (66%) in RIST, respectively. All patients received prophylactic antifungal agents; 89% fluconazole. Number of patients who received the dosage recommended in the CDC guidelines (400 mg/day) was 135 (42%) in conventional transplant and 34 (30%) in RIST (P=0.037). Number of patients who received fluconazole until engraftment and beyond day 75 in conventional transplant vs RIST was, respectively, 324 (100%) vs 109 (97%), and 39 (12%) vs 18 (16%), with no significant difference between the two groups. A total of 37 patients (4.0%) were diagnosed with deep fungal infections; autologous transplantation (0.03%), conventional transplantation (6.0%) and RIST (7.1%). Wide variations in antifungal prophylaxis practice according to the type of transplant and the institutions, and deep fungal infection remain significant problems in RIST.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wingard JR . Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant 1999; 5: 55–68.
Cornely OA, Ullmann AJ, Karthaus M . Evidence-based assessment of primary antifungal prophylaxis in patients with hematologic malignancies. Blood 2003; 101: 3365–3372.
Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 2000; 6 (6a): 659–713; 715; 717–727; quiz 729–733.
Goodman JL, Winston DJ, Greenfield RA et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326: 845–851.
Slavin MA, Osborne B, Adams R et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation – a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545–1552.
Marr KA, Seidel K, Slavin MA et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96: 2055–2061.
Marr KA, Seidel K, White TC et al. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis 2000; 181: 309–316.
Wingard JR, Merz WG, Rinaldi MG et al. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med 1991; 325: 1274–1277.
Kami M, Machida U, Okuzumi K et al. Effect of fluconazole prophylaxis on fungal blood cultures: an autopsy-based study involving 720 patients with haematological malignancy. Br J Haematol 2002; 117: 40–46.
Loo VG, Bertrand C, Dixon C et al. Control of construction-associated nosocomial aspergillosis in an antiquated hematology unit. Infect Control Hosp Epidemiol 1996; 17: 360–364.
Oren I, Haddad N, Finkelstein R et al. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66: 257–262.
Anaissie EJ, Stratton SL, Dignani MC et al. Pathogenic molds (including Aspergillus species) in hospital water distribution systems: a 3-year prospective study and clinical implications for patients with hematologic malignancies. Blood 2003; 101: 2542–2546.
Marr KA, Carter RA, Boeckh M et al. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100: 4358–4366.
McSweeney PA, Niederwieser D, Shizuru JA et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001; 97: 3390–3400.
Winston DJ, Maziarz RT, Chandrasekar PH et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 2003; 138: 705–713.
Herbrecht R, Denning DW, Patterson TF et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002; 347: 408–415.
Bornhauser M, Thiede C, Platzbecker U et al. Dose-reduced conditioning and allogeneic hematopoietic stem cell transplantation from unrelated donors in 42 patients. Clin Cancer Res 2001; 7: 2254–2262.
Barker JN, Weisdorf DJ, DeFor TE et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood 2003; 102: 1915–1919.
Childs R, Chernoff A, Contentin N et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 2000; 343: 750–758.
Mielcarek M, Martin PJ, Leisenring W et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 2003; 102: 756–762.
Fukuda T, Boeckh M, Carter RA et al. Invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplantation after nonmyeloablative conditioning: risks and outcomes. Blood 2003; e-pub.
Hagen EA, Stern H, Porter D et al. High rate of invasive fungal infections following nonmyeloablative allogeneic transplantation. Clin Infect Dis 2003; 36: 9–15.
Kojima R, Kusumi E, Nannya Y et al. Invasive pulmonary aspergillosis (IPA) after reduced-intensity hematopoietic stem cell transplantation (RIST). Blood 2002; 100: 438b.
Ascioglu S, Rex JH, de Pauw B et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
USPHS/IDSA. 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. U.S. Public Health Service (USPHS) and Infectious Diseases Society of America (IDSA). MMWR Recomm Rep 1999; 48 (RR-10): 1–59, 61–66.
Kanda Y, Kami M, Matsuyama T et al. Plasma concentration of itraconazole in patients receiving chemotherapy for hematological malignancies: the effect of famotidine on the absorption of itraconazole. Hematol Oncol 1998; 16: 33–37.
Yoshida M, Tsubaki K, Kobayashi T et al. Infectious complications during remission induction therapy in 577 patients with acute myeloid leukemia in the Japan Adult Leukemia Study Group studies between 1987 and 1991. Int J Hematol 1999; 70: 261–267.
Kojima R, Kusumi E, Nannya Y et al. Invasive pulmonary aspergillosis (IPA) after reduced-intensity hematopoietic stem cell transplantation (RIST). Blood 2002; 100: 438b.
Ruiz-Arguelles GJ, Gomez-Almaguer D, Ruiz-Arguelles A et al. Results of an outpatient-based stem cell allotransplant program using nonmyeloablative conditioning regimens. Am J Hematol 2001; 66: 241–244.
Gonzalez-Ryan L, Haut PR, Coyne K et al. Developing a pediatric outpatient transplantation program. The Children's Memorial Hospital experience. Front Biosci 2001; 6: G1–G5.
Rotstein C, Bow EJ, Laverdiere M et al. Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis 1999; 28: 331–340.
White A, Goetz MB . Azole-resistant Candida albicans: report of two cases of resistance to fluconazole and review. Clin Infect Dis 1994; 19: 687–692.
Dranitsaris G, Phillips P, Rotstein C et al. Economic analysis of fluconazole versus amphotericin B for the treatment of candidemia in non-neutropenic patients. Pharmacoeconomics 1998; 13: 509–518.
Marr KA, Hoyle M, Balajee A et al. Itraconazole vs fluconazole for antifungal prophylaxis in allogeneic HSCT recipients: results of a randomized trial. Blood 2002; 100: 215a.
Kami M, Sawada Y, Mori S et al. Serum levels of fluconazole in patients after cytotoxic chemotherapy for hematological malignancy. Am J Hematol 2001; 66: 85–91.
MacMillan ML, Goodman JL, DeFor TE et al. Fluconazole to prevent yeast infections in bone marrow transplantation patients: a randomized trial of high versus reduced dose, and determination of the value of maintenance therapy. Am J Med 2002; 112: 369–379.
Kami M, Tanaka Y, Kanda Y et al. Computed tomographic scan of the chest, latex agglutination test and plasma (1-3)-beta-D-glucan assay in early diagnosis of invasive pulmonary aspergillosis: a prospective study of 215 patients. Haematologica 2000; 85: 745–752.
Obayashi T, Yoshida M, Mori T et al. Plasma (1 → 3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 1995; 345: 17–20.
Maertens J, Verhaegen J, Lagrou K et al. Screening for circulating galactomannan as a noninvasive diagnostic tool for invasive aspergillosis in prolonged neutropenic patients and stem cell transplantation recipients: a prospective validation. Blood 2001; 97: 1604–1610.
Acknowledgements
This study was supported by a Grant-in-Aid for scientific research from the Ministry of Health, Labor and Welfare.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
This study was conducted at the following institutions under the auspices of the following investigators in Japan:
Masaya Mukai (Sapporo City General Hospital, Hokkaido), Aoyagi Yumei (Dokkyo University School of Medicine, Tochigi), Kazuaki Yakushiji (Kurume University School of Medicine, Fukuoka), Hisashi Tsurumi (Gifu University School of Medicine, Gifu), Yukiyoshi Moriuchi (Sasebo City General Hospital, Nagasaki), Shin Imamura (Fukui Medical University, Fukui), Hiroatu Ago (Shimane Prefectural Central Hospital, Shimane), Sachiko Suzuki (Hakodate Central General Hospital, Hokkaido), Akira Yokota (Chiba Aoba Municipal Hospital, Chiba), Hideki Mitsui (Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka), Yuichi Ishikawa (Chukyo Hospital, Aichi), Yasushi Takamatsu (Fukuoka University School of Medicine, Fukuoka), Kazutaka Sunami (National Okayama Medical Center, Okayama), Nobuo Masauzi (Hakodate Manucipal Hospital, Hokkaido), Tsunehiko Komatsu (Tsukuba Memorial Hospital, Ibaraki), Tadao Ishida (Sapporo Medical University School of Medicine, Hokkaido), Hideo Hyodo (Hiroshima University Research Institute for Radiation Biology and Medicine, Hiroshima), Hiroto Kaneko (Aiseikai-Yamashina Hospital, Kyoto), Hidetaka Takimoto (Kochi Municipal Central Hospital, Kochi), Tadasu Tobita (Yakizu City Hospital, Shiga), Tsutomu Kato (Toyama Medical and Pharmaceutical University, Toyama), Eiichi Ohtsuka (Oita Medical University, Oita), Hideo Kimura (Kita-Fukushima Medical Center, Fukushima), Kimiharu Uozumi (Kagoshima University, Faculty of Medicine, Kagoshima), Toshiro Ito (Shinshu University, The Department of Medicine, Nagano), Masami Inoue (Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka), Yoshinao Yamamoto (Kishiwada City Hospital, Osaka), Shoichi Doi (Kyoto-Katsura Hospital, Kyoto), Yasuhiko Miyazaki (Kansai Medical University, Osaka), Jyunichi Yamagami (Saitama Prefectural Children Medical Center, Saitama), Toshiharu Tamaki (Rinku General Medical Center, Osaka), Yujiro Yamano (Kyushu Kosei-Nenkin Hospital, Fukuoka), Makoto Hirokawa (Akita University School of Medicine, Akita), Shuji Ozaki (Tokushima University Hospital, Tokushima), Koichiro Muta (Kyushu University Graduate School of Medical Science, Fukuoka), Shuichi Hanada (National Kyushu Cardiovascular Center, Fukuoka), Nobuhiko Uoshima (Matsushita Memorial Hospital, Osaka), Hiroyuki Tsuda (Kumamoto City Hospital, Kumamoto), Chihiro Shimazaki (Kyoto Prefectural University of Medicine, Kyoto), Atsushi Wakita (Nagoya City University Graduate School of Medical Sciences, Aichi), Tetsuya E Tanimoto (Kyushu University Faculty of Medicine, Fukuoka), Masaki Ri (Shizuoka Saiseikai General Hospital, Shizuoka), Eisaburo Sueoka (Saga Medical School, Saga), Jyunichi Tsukada (University of Occupational and Environmental Health School of Medicine, Fukuoka), Akihiro Ihara (National Hospital Kure Medical Center, Hiroshima).
Rights and permissions
About this article
Cite this article
Imataki, O., Kami, M., Kim, SW. et al. A nationwide survey of deep fungal infections and fungal prophylaxis after hematopoietic stem cell transplantation in Japan. Bone Marrow Transplant 33, 1173–1179 (2004). https://doi.org/10.1038/sj.bmt.1704526
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704526
Keywords
This article is cited by
-
Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies
International Journal of Hematology (2012)
-
Antimicrobial therapy of febrile complications after high-dose chemotherapy and autologous hematopoietic stem cell transplantation—guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO)
Annals of Hematology (2012)
-
Medical cost analysis for antifungal prophylaxis in neutropenic patients with hematological malignancies: a systematic simulation analysis
Supportive Care in Cancer (2011)
-
Invasive Fungal Infection in Haematopoietic Stem Cell Transplant Recipients: Epidemiology from the Transplant Physician’s Viewpoint
Mycopathologia (2009)
-
Comparison between reduced intensity and conventional myeloablative allogeneic stem-cell transplantation in patients with hematologic malignancies aged between 50 and 59 years
Bone Marrow Transplantation (2005)