Summary:
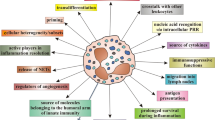
Transfusion-associated graft-versus-host disease (TA-GVHD) is a serious complication of blood component transfusion therapy. Currently, cellular blood components for patients recognized at risk for TA-GVHD are irradiated prior to transfusion in order to prevent this complication. Considerable progress has been made in elucidating the pathophysiology of this highly morbid complication, but questions as to which patients are at risk and what is the most robust technology to prevent TA-GVHD remain. As new technologies for inactivating or modulating leukocyte function are introduced, the question of how to evaluate these technologies becomes relevant. Over the past two decades, a number of research groups have explored technology to inactivate infectious pathogens and leukocytes contaminating cellular blood components. Few clinicians have an in-depth understanding of the methods or the criteria for selection of how to approach new technologies for leukocyte inactivation with potential to replace current methods. This mini review focuses on the salient aspects of current and evolving technology for prevention of TA-GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anderson KC, Weinstein HJ . Transfusion-associated graft-versus-host disease. N Eng J Med 1990; 323: 315–321.
Ohto H, Anderson KC . Survey of transfusion-associated graft-versus-host disease in immunocompetent recipients. Transfusion Med Rev 1996; 10: 31–43.
Hathaway WE, Githens JA, Blackburn JR et al. Aplastic anemia, histiocytosis, and erythroderma in immunologically deficient children. N Engl J Med 1965; 273: 953.
vonFliedner V, Higby DJ, Kim U . Graft-versus-host reaction following blood product transfusion. Am J Med 1972; 72: 951.
Brubaker DB . Immunopathogenic mechanims of post-transfusion graft versus host disease. Proc Soc Exp Biol Med 1993; 202: 122–147.
Fearon TC, Luban NLC . Practical dosimetric aspects of blood and blood product irradiation. Transfusion 1986; 26: 457–459.
Crowley JP, Skrabut EM, Valeri CR . Immunocompetent lymphocytes in previously frozen washed red cells. Vox Sanguinis 1974; 26: 513–517.
Akahoshi M, Takanashi M, Masuda M . A case of transfusion-associated graft-versus-host disease not prevented by white cell-reduction filters. Transfusion 1992; 32: 169–172.
Hayashi H, Nishiuchi T, Tamura H . Transfusion-associated graft-versus-host disease caused by leukocyte filtered stored blood. Anesthesiology 1993; 79: 1419–1421.
CBER F. Gamma Irradiation of Blood and Blood Components: A Pilot Program for Licensing. Food and Drug Administration Center for Biologics Evaluation and Research (CBER): Rockville, 2000, 1–12.
Wieding JU, Vehmeyer K, Riggert J et al. Fresh–frozen plasma contains proliferable stem cells. Transfusion 1993; 33 (Suppl 9S): 53S.
Sprent J, Anderson RE, Miller JFAP . Radiosensitivity of T and B lymphocytes. II. Effect of radiation on response of T cells to alloantigens. Eur J Immunol 1974; 4: 204.
Dobrynski W, Thibodeau S, Truitt RL et al. Third-party-mediated graft rejection and graft-versus-host disease after T-cell-depleted bone marrow transplantation as demonstrated by hypervariable DNA probes and HLA-DR polymorphism. Blood 1989; 74: 2285.
Sproul AM, Chalmers EA, Mills KI . Third part mediated graft rejection despite irradiation of blood products. Br J Haematol 1993; 80: 251–252.
Lowenthal RM, Challis DR, Griffiths AE . Transfusion-associated graft-versus-host disease: report of an occurrence following administration of irradiated blood. Transfusion 1993; 33: 524–529.
Barbara J . The rationale for pathogen inactivation treatment of platelet components – introduction. Sem Hematol 2001; 38 (Suppl 11): 1–3.
Rosen NR, Wiedner JG, Boldt HD et al. Prevention of transfusion-associated graft-versus-host disease: selection of an adequate dose of gamma irradiation. Transfusion 1993; 33: 125.
Kanter MH . Transfusion-associated graft-versus-host disease: do transfusions from second-degree relatives pose a greater risk than those from first-degree relatives? Transfusion 1992; 32: 323–327.
Benson K, Marks AR, Marshall MJ . Fatal graft-versus-host disease associated with transfusions of HLA-matched, HLA homozygous platelets from unrelated donors. Transfusion 1994; 34: 432–437.
Lee TH, Paglieroni T, Ohto H, Holland PV, Busch MP . Survival of donor leukocyte subpopulations in immunocompetent transfusion recipients: frequent long-term microchimerism in severe trauma patients. Blood 1999; 93: 3127–3139.
Rosen NR, Wiedner JG, Bold HD . Prevention of transfusion-associated graft-versus-host disease: selection of an adequate dose of gamma irradiation. Transfusion 1993; 33: 125–127.
Luban NLC . Prevention of transfusion-associated graft-versus-host disease by inactivation of T cells in platelet components. Sem Hematol 2001; 38 (Suppl 11): 34–45.
Pelszynski MM, Moroff G, Luban NLC et al. Effect of gamma irradiation on T-cell inactivation as assessed by limiting dilution Analysis: Implications for Preventing transfusion associated graft vs host disease. Blood 1994; 83: 1683.
Seghatchian MJ, Stivala JFA . Effect of 25 Gy gamma irradiation on storage stability of three types of platelet concentrates: a comparative analysis with paired controls and random preparation. Transfusion Sci 1995; 16: 121–129.
Edelson R, Berger C, Gasparro F et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. N Engl J Med 1987; 316: 297–303.
French LE, Alcindor T, Shapiro M et al. Identification of amplified clonal T cell populations in the blood of patients with chronic graft-versus-host disease: positive correlation with response to photopheresis. Bone Marrow Transplant 2002; 30: 509–515.
Dodd RY, Moroff G, Wagner S et al. Inactivation of viruses in platelet suspensions that retain their in vitro characteristics: comparison of psoralen–ultraviolet A and merocyanine 540-visible light methods. Transfusion 1991; 31: 483–490.
Lin L, Wiesehahn GP, Morel PA et al. Use of 8-methoxypsoralen and long wavelength ultraviolet radiation for decontamination of platelet concentrates. Blood 1989; 74: 517–525.
Lin L, Londe H, Hanson CV et al. Psoralen photochemical inactivation of cell-free HIV-1 in platelet concentrates is correlated with the inhibition of reverse transcription using an RT/PCR assay. Blood 1992; 80 (Suppl 1): 223a.
Wagner SJ, Bardossy L, Moroff G et al. Assessment of the hemostatic effectiveness of human platelets treated with aminomethyltrimethyl psoralen and UVA light using a rabbit ear bleeding time technique. Blood 1993; 82: 3489–3492.
Lin L, Cook DN, Wiesehahn GP et al. Photochemical inactivation of viruses and bacteria in platelet concentrates by use of a novel psoralen and long-wavelength ultraviolet light. Transfusion 1997; 37: 423–435.
van Rhenen DJ, Vermeij J, Mayaudon V et al. In vitro platelet function of S59 photochemically treated platelet concentrates. Transfusion 1997; 37 (Suppl): 14s.
Grass JA, Hei DJ, Metchette K et al. Inactivation of leukocytes in platelet concentrates by psoralen plus UVA. Blood 1998; 91: 2180–2188.
Hei DJ, Grass J, Lin L et al. Elimination of cytokine production in stored platelet concentrate aliquots by photochemical treatment with psoralen plus ultraviolet A light. Transfusion 1999; 39: 239–248.
Grass JA, Wafa T, Reames A et al. Prevention of transfusion-associated graft-versus-host disease by photochemical treatment. Blood 1999; 93: 3140–3147.
Knutson F, Alfonso R, Dupuis K et al. Photochemical inactivation of bacteria and HIV in buffy-coat-derived platelet concentrates under conditions that preserve in vitro platelet function. Vox Sanguinis 2000; 78: 209–216.
vanRhenen D, Gulliksson H, Cazenave JP et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood 2003; 101: 2426–2433.
Wollowitz S . Fundamentals of the psoralen-based Helinx technology for inactivation of infectious pathogens and leukocytes in platelets and plasma. Sem Hematol 2001; 38 (Suppl 11): 4–11.
vanRhenen DJ, Vermeij J, Mayaudon V et al. Functional characteristics of S-59 photochemically treated platelet concentrates derived from buffy coats. Vox Sanguinis 2000; 79: 206–214.
Przepiorka D, LeParc GF, Stovall MA et al. Use of irradiated blood components. Am J Clin Pathol 1995; 106: 6.
Fiebig E, Hirschkorn DF, Maino VC et al. Assessment of donor T-cell function in cellular blood components by the CD69 induction assay: effects of storage, gamma irradiation, and photochemical treatment. Transfusion 2000; 40: 761–770.
Fast LD, DiLeone G, Edson CM et al. PEN 110 treatment funtionally inactivates the PBMNCs present in RBC units: comparison to the effects of exposure to gamma irradiation. Transfusion 2002; 42: 1318–1325.
Setlow RB, Setlow JK . Effect of radiation on polynucleotides. In: Baldwin ML JL (ed.) Irradiation of Blood Components, Volume: 1–30 AABB Press: Bethesda, 1992.
Ferrara J, Burakoff SJ . The pathophysiology of acute graft vs host disease in a murine bone marrow transplant model. In: Burakoff SJ, Deeg HJ, Ferrara J, Atkinson K (eds). Graft Versus Host Disease: Immunology, Pathophysiology, and Treatment. Marcel Dekker, Inc.: New York, 1990, 9–29.
Fast LD, Valeri CR, Crowley JP . Immune responses to major histocompatibility complex homozygous cells in murine F 1 hybrid recipients: implications for transfusion- associated graft-versus-host disease. Blood 1995; 86: 3090.
Grass J, Delmonte J, Wages D et al. Prevention of transfusion-associated graft vs host disease (TA-GVHD) in immunocompromised mice by photochemical treatment (PCT) of donor T cells. Blood 1997; 90 (Suppl 1): 207a.
Truitt RL, Johnson BD, Hanke C et al. Photochemical treatment with S-59 psoralen and ultraviolet A light to control the fate of naive or primed T lymphocytes in vivo after allogeneic bone marrow transplantation. J Immunol 1999; 163: 5145–5156.
Fast LD, DiLeone G, Edson CM et al. Inhibition of murine GVHD by PEN 110 treatment. Transfusion 2002; 42: 1326–1332.
vanRhenen D, Gulliksson H, Pamphilon D et al. S-59 Helinx photochemically treated platelets(plt) are safe and effective for support of thrombocytopenia: results of the EUROSPRITE Phase 3 trial. Blood 2000; 96: 819a.
Conlan M, Lin JS, Flament J, Lin L, Corash L . INTERCEPT platelets treated with Helinx technology to inactivate T-cells prevented transfusion-associated graft-versus-host disease (TA-GVHD): results of 3 clinical trials. VIII European Congress of the International Society of Blood Transfusion 2003, Istanbul, Turkey International Society of Blood Transfusion, 2003, p 22.
Deeg HJ, Graham TC, Gerhard-Miller L et al. Prevention of transfusion-induced graft-versus-host disease in dogs by ultraviolet irradiation. Blood 1989; 74: 2592–2595.
Lin L, Londe H, Hanson CV et al. Photochemical inactivation of cell-associated human immunodeficiency virus in platelet concentrates. Blood 1993; 82: 292–297.
Moroff G, Wagner S, Benade L et al. Factors influencing virus inactivation and retention of platelet properties following treatment with aminomethyltrimethylpsoralen and ultraviolet. A light. Blood Cells 1992; 18: 43–56.
Prodouz KN, Lytle CD, Keville EA et al. Inhibition by albumin of merocyanine 540-mediated photosensitization of platelets and viruses. Transfusion 1991; 31: 415–422.
Rywkin S, Ben-Hur E, Malik Z et al. New phthalocyanines for photodynamic virus inactivation in red blood cell concentrates. Photochem Photobiol 1994; 60: 165–170.
Skripchenko AA, Wagner SJ . Inactivation of WBCs in RBC suspensions by photoactive phenothiazine dyes: comparison of dimethylmethylene blue and MB. Transfusion 2000; 40: 968–975.
Hanson D, Propst M, Dupuis K et al. High titer leukocyte inactivation in INTERCEPT red blood cells (RBC). Transfusion Clin Biol 2001; 8 (Suppl 1): 45s.
Goodrich RP . The use of riboflavin for the inactivation of pathogens in blood products. Vox Sanguinis 2000; 78 (Suppl 2): 211–215.
Mohr H, Redecker-Klein A . Inactivation of pathogens in platelet concentrates by uisng a two-step procedure. Vox Sanguinis 2003; 84: 96–104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corash, L., Lin, L. Novel processes for inactivation of leukocytes to prevent transfusion-associated graft-versus-host disease. Bone Marrow Transplant 33, 1–7 (2004). https://doi.org/10.1038/sj.bmt.1704284
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704284
Keywords
This article is cited by
-
Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming
Nature Communications (2016)
-
Safety and clinical efficacy of platelet components prepared with pathogen inactivation in routine use for thrombocytopenic patients
Annals of Hematology (2011)